Plasticity of hippocampal stem/progenitor cells to enhance neurogenesis in response to kainate-induced injury is lost by middle age
- PMID: 18241325
- PMCID: PMC3612497
- DOI: 10.1111/j.1474-9726.2007.00363.x
Plasticity of hippocampal stem/progenitor cells to enhance neurogenesis in response to kainate-induced injury is lost by middle age
Abstract
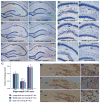
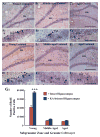
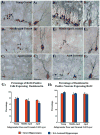
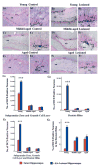
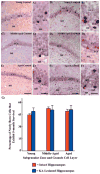
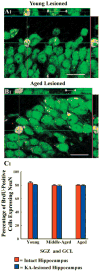
A remarkable up-regulation of neurogenesis through increased proliferation of neural stem/progenitor cells (NSCs) is a well-known plasticity displayed by the young dentate gyrus (DG) following brain injury. To ascertain whether this plasticity is preserved during aging, we quantified DG neurogenesis in the young adult, middle-aged and aged F344 rats after kainic acid induced hippocampal injury. Measurement of new cells that are added to the dentate granule cell layer (GCL) between post-injury days 4 and 15 using 5'-bromodeoxyuridine labeling revealed an increased addition of new cells in the young DG but not in the middle-aged and aged DG. Quantification of newly born neurons using doublecortin immunostaining also demonstrated a similar trend. Furthermore, the extent of ectopic migration of new neurons into the dentate hilus was dramatically increased in the young DG but was unaltered in the middle-aged and aged DG. However, there was no change in neuronal fate-choice decision of newly born cells following injury in all age groups. Similarly, comparable fractions of new cells that are added to the GCL after injury exhibited 5-month survival and expressed the mature neuronal marker NeuN, regardless of age or injury at the time of their birth. Thus, hippocampal injury does not adequately stimulate NSCs in the middle-aged and aged DG, resulting in no changes in neurogenesis after injury. Interestingly, rates of both neuronal fate-choice decision and long-term survival of newly born cells remain stable with injury in all age groups. These results underscore that the ability of the DG to increase neurogenesis after injury is lost as early as middle age.
Figures
References
-
- Abdel-Rahman A, Rao MS, Shetty AK. Nestin expression in hippocampal astrocytes after injury depends on the age of the hippocampus. Glia. 2004;47:299– 313. - PubMed
-
- Aimone JB, Wiles J, Gage FH. Potential role for adult neurogenesis in the encoding of time in new memories. Nat Neurosci. 2006;9:723– 727. - PubMed
-
- Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124:319– 335. - PubMed
-
- Bernal GM, Peterson DA. Neural stem cells as therapeutic agents for age-related brain repair. Aging Cell. 2004;3:345– 351. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

