Brca1 breast tumors contain distinct CD44+/CD24- and CD133+ cells with cancer stem cell characteristics
- PMID: 18241344
- PMCID: PMC2374965
- DOI: 10.1186/bcr1855
Brca1 breast tumors contain distinct CD44+/CD24- and CD133+ cells with cancer stem cell characteristics
Abstract
Introduction: Whether cancer stem cells occur in BRCA1-associated breast cancer and contribute to therapeutic response is not known.
Methods: We generated and characterized 16 cell lines from five distinct Brca1deficient mouse mammary tumors with respect to their cancer stem cell characteristics.
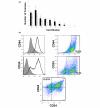
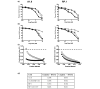
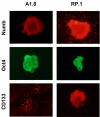
Results: All cell lines derived from one tumor included increased numbers of CD44+/CD24- cells, which were previously identified as human breast cancer stem cells. All cell lines derived from another mammary tumor exhibited low levels of CD44+/CD24- cells, but they harbored 2% to 5.9% CD133+ cells, which were previously associated with cancer stem cells in other human and murine tumors. When plated in the absence of attachment without presorting, only those cell lines that were enriched in either stem cell marker formed spheroids, which were further enriched in cells expressing the respective cancer stem cell marker. In contrast, cells sorted for CD44+/CD24- or CD133+ markers lost their stem cell phenotype when cultured in monolayers. As few as 50 to 100 CD44+/CD24- or CD133+ sorted cells rapidly formed tumors in nonobese diabetic/severe combined immunodeficient mice, whereas 50-fold to 100-fold higher numbers of parental or stem cell depleted cells were required to form few, slow-growing tumors. Expression of stem cell associated genes, including Oct4, Notch1, Aldh1, Fgfr1, and Sox1, was increased in CD44+/CD24- and CD133+ cells. In addition, cells sorted for cancer stem cell markers and spheroid-forming cells were significantly more resistant to DNA-damaging drugs than were parental or stem cell depleted populations, and they were sensitized to the drugs by the heat shock protein-90 inhibitor 17-DMAG (17-dimethylaminoethylamino-17-demethoxygeldanamycin hydrochloride).
Conclusion: Brca1-deficient mouse mammary tumors harbor heterogeneous cancer stem cell populations, and CD44+/CD24- cells represent a population that correlates with human breast cancer stem cells.
Figures



Comment in
-
Cancer stem cell heterogeneity in hereditary breast cancer.Breast Cancer Res. 2008;10(2):105. doi: 10.1186/bcr1990. Epub 2008 Apr 16. Breast Cancer Res. 2008. PMID: 18423071 Free PMC article.
Similar articles
-
Prolonged drug selection of breast cancer cells and enrichment of cancer stem cell characteristics.J Natl Cancer Inst. 2010 Nov 3;102(21):1637-52. doi: 10.1093/jnci/djq361. Epub 2010 Oct 8. J Natl Cancer Inst. 2010. PMID: 20935265 Free PMC article.
-
The clinicopathological and prognostic significance of CD24, CD44, CD133, ALDH1 expressions in invasive ductal carcinoma of the breast: CD44/CD24 expression in breast cancer.Pathol Res Pract. 2015 Oct;211(10):740-7. doi: 10.1016/j.prp.2015.05.011. Epub 2015 Jun 1. Pathol Res Pract. 2015. PMID: 26298632
-
CD44+ CD133+ population exhibits cancer stem cell-like characteristics in human gallbladder carcinoma.Cancer Biol Ther. 2010 Dec 1;10(11):1182-90. doi: 10.4161/cbt.10.11.13664. Epub 2010 Dec 1. Cancer Biol Ther. 2010. PMID: 20948317
-
Breast cancer stem cells and intrinsic subtypes: controversies rage on.Curr Stem Cell Res Ther. 2009 Jan;4(1):50-60. doi: 10.2174/157488809787169110. Curr Stem Cell Res Ther. 2009. PMID: 19149630 Review.
-
A review of the racial heterogeneity of breast cancer stem cells.Gene. 2021 Sep 5;796-797:145805. doi: 10.1016/j.gene.2021.145805. Epub 2021 Jun 29. Gene. 2021. PMID: 34197949 Review.
Cited by
-
In vivo molecular imaging of cancer stem cells.Am J Nucl Med Mol Imaging. 2014 Dec 15;5(1):14-26. eCollection 2015. Am J Nucl Med Mol Imaging. 2014. PMID: 25625023 Free PMC article. Review.
-
"A novel in vivo model for the study of human breast cancer metastasis using primary breast tumor-initiating cells from patient biopsies".BMC Cancer. 2012 Jan 10;12:10. doi: 10.1186/1471-2407-12-10. BMC Cancer. 2012. PMID: 22233382 Free PMC article.
-
Cancer stem cell hypotheses: impact on modern molecular physiology and pharmacology research.J Biosci. 2011 Dec;36(5):957-61. doi: 10.1007/s12038-011-9155-5. J Biosci. 2011. PMID: 22116294 Review.
-
Strategies for isolating and enriching cancer stem cells: well begun is half done.Stem Cells Dev. 2013 Aug 15;22(16):2221-39. doi: 10.1089/scd.2012.0613. Epub 2013 May 9. Stem Cells Dev. 2013. PMID: 23540661 Free PMC article. Review.
-
Cell specific CD44 expression in breast cancer requires the interaction of AP-1 and NFκB with a novel cis-element.PLoS One. 2012;7(11):e50867. doi: 10.1371/journal.pone.0050867. Epub 2012 Nov 30. PLoS One. 2012. PMID: 23226410 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

