Caenorhabditis elegans EAK-3 inhibits dauer arrest via nonautonomous regulation of nuclear DAF-16/FoxO activity
- PMID: 18241854
- PMCID: PMC2350227
- DOI: 10.1016/j.ydbio.2007.12.032
Caenorhabditis elegans EAK-3 inhibits dauer arrest via nonautonomous regulation of nuclear DAF-16/FoxO activity
Abstract
Insulin regulates development, metabolism, and lifespan via a conserved PI3K/Akt pathway that promotes cytoplasmic sequestration of FoxO transcription factors. The regulation of nuclear FoxO is poorly understood. In the nematode Caenorhabditis elegans, insulin-like signaling functions in larvae to inhibit dauer arrest and acts during adulthood to regulate lifespan. In a screen for genes that modulate C. elegans insulin-like signaling, we identified eak-3, which encodes a novel protein that is specifically expressed in the two endocrine XXX cells. The dauer arrest phenotype of eak-3 mutants is fully suppressed by mutations in daf-16/FoxO, which encodes the major target of C. elegans insulin-like signaling, and daf-12, which encodes a nuclear receptor regulated by steroid hormones known as dafachronic acids. eak-3 mutation does not affect DAF-16/FoxO subcellular localization but enhances expression of the direct DAF-16/FoxO target sod-3 in a daf-16/FoxO- and daf-12-dependent manner. eak-3 mutants have normal lifespans, suggesting that EAK-3 decouples insulin-like regulation of development and longevity. We propose that EAK-3 activity in the XXX cells promotes the synthesis and/or secretion of a hormone that acts in parallel to AKT-1 to inhibit the expression of DAF-16/FoxO target genes. Similar hormonal pathways may regulate FoxO target gene expression in mammals.
Figures
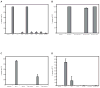
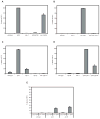
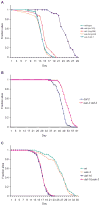
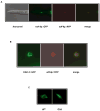
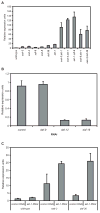
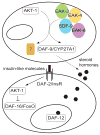

Similar articles
-
Effects of Caenorhabditis elegans sgk-1 mutations on lifespan, stress resistance, and DAF-16/FoxO regulation.Aging Cell. 2013 Oct;12(5):932-40. doi: 10.1111/acel.12120. Epub 2013 Jul 19. Aging Cell. 2013. PMID: 23786484 Free PMC article.
-
Activated AKT/PKB signaling in C. elegans uncouples temporally distinct outputs of DAF-2/insulin-like signaling.BMC Dev Biol. 2006 Oct 4;6:45. doi: 10.1186/1471-213X-6-45. BMC Dev Biol. 2006. PMID: 17020605 Free PMC article.
-
EAK proteins: novel conserved regulators of C. elegans lifespan.Aging (Albany NY). 2010 Oct;2(10):742-7. doi: 10.18632/aging.100214. Aging (Albany NY). 2010. PMID: 20975207 Free PMC article.
-
DAF-16: FOXO in the Context of C. elegans.Curr Top Dev Biol. 2018;127:1-21. doi: 10.1016/bs.ctdb.2017.11.007. Epub 2018 Feb 2. Curr Top Dev Biol. 2018. PMID: 29433733 Review.
-
The search for DAF-16/FOXO transcriptional targets: approaches and discoveries.Exp Gerontol. 2006 Oct;41(10):910-21. doi: 10.1016/j.exger.2006.06.040. Epub 2006 Aug 24. Exp Gerontol. 2006. PMID: 16934425 Review.
Cited by
-
Functional divergence of dafachronic acid pathways in the control of C. elegans development and lifespan.Dev Biol. 2010 Apr 15;340(2):605-12. doi: 10.1016/j.ydbio.2010.02.022. Epub 2010 Feb 21. Dev Biol. 2010. PMID: 20178781 Free PMC article.
-
Effects of Caenorhabditis elegans sgk-1 mutations on lifespan, stress resistance, and DAF-16/FoxO regulation.Aging Cell. 2013 Oct;12(5):932-40. doi: 10.1111/acel.12120. Epub 2013 Jul 19. Aging Cell. 2013. PMID: 23786484 Free PMC article.
-
Untangling Longevity, Dauer, and Healthspan in Caenorhabditis elegans Insulin/IGF-1-Signalling.Gerontology. 2018;64(1):96-104. doi: 10.1159/000480504. Epub 2017 Sep 22. Gerontology. 2018. PMID: 28934747 Free PMC article.
-
EAK-7 controls development and life span by regulating nuclear DAF-16/FoxO activity.Cell Metab. 2010 Jul 7;12(1):30-41. doi: 10.1016/j.cmet.2010.05.004. Cell Metab. 2010. PMID: 20620993 Free PMC article.
-
Pharmacologic targeting of sirtuin and PPAR signaling improves longevity and mitochondrial physiology in respiratory chain complex I mutant Caenorhabditis elegans.Mitochondrion. 2015 May;22:45-59. doi: 10.1016/j.mito.2015.02.005. Epub 2015 Mar 3. Mitochondrion. 2015. PMID: 25744875 Free PMC article.
References
-
- Ashrafi K, Chang FY, Watts JL, Fraser AG, Kamath RS, Ahringer J, Ruvkun G. Genome-wide RNAi analysis of Caenorhabditis elegans fat regulatory genes. Nature. 2003;421:268–72. - PubMed
-
- Barthel A, Schmoll D, Unterman TG. FoxO proteins in insulin action and metabolism. Trends Endocrinol Metab. 2005;16:183–9. - PubMed
-
- Berdichevsky A, Viswanathan M, Horvitz HR, Guarente L. C. elegans SIR-2.1 interacts with 14-3-3 proteins to activate DAF-16 and extend life span. Cell. 2006;125:1165–77. - PubMed
-
- Berman JR, Kenyon C. Germ-cell loss extends C. elegans life span through regulation of DAF-16 by kri-1 and lipophilic-hormone signaling. Cell. 2006;124:1055–68. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

