Affinity makes the difference: nonselective interaction of the UBA domain of Ubiquilin-1 with monomeric ubiquitin and polyubiquitin chains
- PMID: 18241885
- PMCID: PMC2323583
- DOI: 10.1016/j.jmb.2007.12.029
Affinity makes the difference: nonselective interaction of the UBA domain of Ubiquilin-1 with monomeric ubiquitin and polyubiquitin chains
Abstract
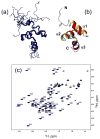
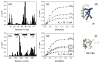
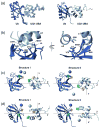
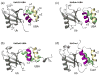
Ubiquilin/PLIC proteins belong to the family of UBL-UBA proteins implicated in the regulation of the ubiquitin-dependent proteasomal degradation of cellular proteins. A human presenilin-interacting protein, ubiquilin-1, has been suggested as potential therapeutic target for treating Huntington's disease. Ubiquilin's interactions with mono- and polyubiquitins are mediated by its UBA domain, which is one of the tightest ubiquitin binders among known ubiquitin-binding domains. Here we report the three-dimensional structure of the UBA domain of ubiquilin-1 (UQ1-UBA) free in solution and in complex with ubiquitin. UQ1-UBA forms a compact three-helix bundle structurally similar to other known UBAs, and binds to the hydrophobic patch on ubiquitin with a K(d) of 20 microM. To gain structural insights into UQ1-UBA's interactions with polyubiquitin chains, we have mapped the binding interface between UQ1-UBA and Lys48- and Lys63-linked di-ubiquitins and characterized the strength of UQ1-UBA binding to these chains. Our NMR data show that UQ1-UBA interacts with the individual ubiquitin units in both chains in a mode similar to its interaction with mono-ubiquitin, although with an improved binding affinity for the chains. Our results indicate that, in contrast to UBA2 of hHR23A that has strong binding preference for Lys48-linked chains, UQ1-UBA shows little or no binding selectivity toward a particular chain linkage or between the two ubiquitin moieties in the same chain. The structural data obtained in this study provide insights into the possible structural reasons for the diversity of polyubiquitin chain recognition by UBA domains.
Figures


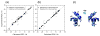


Similar articles
-
Structural analysis of the UBA domain of X-linked inhibitor of apoptosis protein reveals different surfaces for ubiquitin-binding and self-association.PLoS One. 2011;6(12):e28511. doi: 10.1371/journal.pone.0028511. Epub 2011 Dec 15. PLoS One. 2011. PMID: 22194841 Free PMC article.
-
Structural determinants for selective recognition of a Lys48-linked polyubiquitin chain by a UBA domain.Mol Cell. 2005 Jun 10;18(6):687-98. doi: 10.1016/j.molcel.2005.05.013. Mol Cell. 2005. PMID: 15949443
-
Rad23 ubiquitin-associated domains (UBA) inhibit 26 S proteasome-catalyzed proteolysis by sequestering lysine 48-linked polyubiquitin chains.J Biol Chem. 2003 Mar 14;278(11):8951-9. doi: 10.1074/jbc.m212841200. J Biol Chem. 2003. PMID: 12643283
-
Structural and functional studies of mutations affecting the UBA domain of SQSTM1 (p62) which cause Paget's disease of bone.Biochem Soc Trans. 2004 Nov;32(Pt 5):728-30. doi: 10.1042/BST0320728. Biochem Soc Trans. 2004. PMID: 15493999 Review.
-
Ubiquitin-binding domains.Biochem J. 2006 Nov 1;399(3):361-72. doi: 10.1042/BJ20061138. Biochem J. 2006. PMID: 17034365 Free PMC article. Review.
Cited by
-
BcL-xL conformational changes upon fragment binding revealed by NMR.PLoS One. 2013 May 23;8(5):e64400. doi: 10.1371/journal.pone.0064400. Print 2013. PLoS One. 2013. PMID: 23717610 Free PMC article.
-
Electrostatic and steric effects underlie acetylation-induced changes in ubiquitin structure and function.Nat Commun. 2022 Sep 16;13(1):5435. doi: 10.1038/s41467-022-33087-1. Nat Commun. 2022. PMID: 36114200 Free PMC article.
-
Ubiquilins Chaperone and Triage Mitochondrial Membrane Proteins for Degradation.Mol Cell. 2016 Jul 7;63(1):21-33. doi: 10.1016/j.molcel.2016.05.020. Epub 2016 Jun 23. Mol Cell. 2016. PMID: 27345149 Free PMC article.
-
Enhanced Purification of Ubiquitinated Proteins by Engineered Tandem Hybrid Ubiquitin-binding Domains (ThUBDs).Mol Cell Proteomics. 2016 Apr;15(4):1381-96. doi: 10.1074/mcp.o115.051839. Mol Cell Proteomics. 2016. PMID: 27037361 Free PMC article.
-
Ubiquilin4 is an adaptor protein that recruits Ubiquilin1 to the autophagy machinery.EMBO Rep. 2013 Apr;14(4):373-81. doi: 10.1038/embor.2013.22. Epub 2013 Mar 5. EMBO Rep. 2013. PMID: 23459205 Free PMC article.
References
-
- Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem. 1998;67:425–479. - PubMed
-
- Di Fiore PP, Polo S, Hofmann K. When ubiquitin meets ubiquitin receptors: a signalling connection. Nat Rev Mol Cell Biol. 2003;4:491–7. - PubMed
-
- Haglund K, Sigismund S, Polo S, Szymkiewicz I, Di Fiore PP, Dikic I. Multiple monoubiquitination of RTKs is sufficient for their endocytosis and degradation. Nat Cell Biol. 2003;5:461–6. - PubMed
-
- Hicke L, Dunn R. Regulation of membrane protein transport by ubiquitin and ubiquitin-binding proteins. Annu Rev Cell Dev Biol. 2003;19:141–72. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

