Regulation of SIRT6 protein levels by nutrient availability
- PMID: 18242175
- PMCID: PMC3263697
- DOI: 10.1016/j.febslet.2008.01.019
Regulation of SIRT6 protein levels by nutrient availability
Abstract
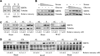
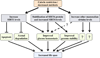
Sirtuins have been shown to regulate life-span in response to nutritional availability. We show here that levels of the mammalian sirtuin, SIRT6, increased upon nutrient deprivation in cultured cells, in mice after fasting, and in rats fed a calorie-restricted diet. The increase in SIRT6 levels is due to stabilization of SIRT6 protein, and not via an increase in SIRT6 transcription. In addition, p53 positively regulates SIRT6 protein levels under standard growth conditions but has no role in the nutrient-dependent regulation of SIRT6. These observations imply that at least two sirtuins are involved in regulation of life-span by nutrient availability.
Figures


References
-
- Blander G, Guarente L. The Sir2 family of protein deacetylases. Annu. Rev. Biochem. 2004;73:417–435. - PubMed
-
- Liszt G, Ford E, Kurtev M, Guarente L. Mouse Sir2 homolog SIRT6 is a nuclear ADP-ribosyltransferase. J. Biol. Chem. 2005;280:21313–21320. - PubMed
-
- Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, Sinclair DA. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. - PubMed
-
- Tissenbaum HA, Guarente L. Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature. 2001;410:227–230. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

