A molecular signature of gastric metaplasia arising in response to acute parietal cell loss
- PMID: 18242217
- PMCID: PMC2857727
- DOI: 10.1053/j.gastro.2007.11.058
A molecular signature of gastric metaplasia arising in response to acute parietal cell loss
Abstract
Background & aims: Loss of gastric parietal cells is a critical precursor to gastric metaplasia and neoplasia. However, the origin of metaplasia remains obscure. Acute parietal cell loss in gastrin-deficient mice treated with DMP-777 leads to the rapid emergence of spasmolytic polypeptide/trefoil factor family 2 (TFF2)-expressing metaplasia (SPEM) from the bases of fundic glands. We now sought to characterize more definitively the pathway for emergence of SPEM.
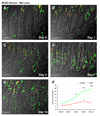
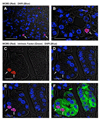
Methods: Emerging SPEM lineages in gastrin-deficient mice treated with DMP-777 were examined for immunolocalization of TFF2, intrinsic factor, and Mist1, and morphologically with electron microscopy. Emerging SPEM was isolated with laser-capture microdissection and RNA was analyzed using gene microarrays. Immunohistochemistry in mouse and human samples was used to confirm up-regulated transcripts.
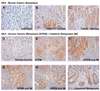
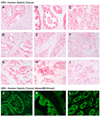
Results: DMP-777-induced SPEM was immunoreactive for TFF2 and the differentiated chief cell markers, Mist1 and intrinsic factor, suggesting that SPEM derived from transdifferentiation of chief cells. Microarray analysis of microdissected SPEM lineages induced by DMP-777 showed up-regulation of transcripts associated with G1/S cell-cycle transition including minichromosome maintenance deficient proteins, as well as a number of secreted factors, including human epididymis 4 (HE4). HE4, which was absent in the normal stomach, was expressed in SPEM of human and mouse and in intestinal metaplasia and gastric cancer in human beings.
Conclusions: Although traditionally metaplasia was thought to originate from normal mucosal progenitor cells, these studies indicate that SPEM evolves through either transdifferentiation of chief cells or activation of a basal cryptic progenitor. In addition, induction of metaplasia elicits the expression of secreted factors, such as HE4, relevant to gastric preneoplasia.
Conflict of interest statement
Figures


References
-
- Pisani P, Bray F, Parkin DM. Estimates of the world-wide prevalence of cancer for 25 sites in the adult population. Int J Cancer. 2002;97:72–81. - PubMed
-
- Parsonnet J, Friedman GD, Vandersteen DP, Chang Y, Vogelman JH, Orentreich N, Sibley RK. Helicobacter pylori infection and the risk of gastric cancer. New Eng.J.Med. 1991;325:1127–1131. - PubMed
-
- Stepan V, Ramamoorthy S, Nitsche H, Zavros Y, Merchant JL, Todisco A. Regulation and function of the sonic hedgehog signal transduction pathway in isolated gastric parietal cells. J Biol Chem. 2005;280:15700–15708. - PubMed
-
- Schmidt PH, Lee JR, Joshi V, Playford RJ, Poulsom R, Wright NA, Goldenring JR. Identification of a metaplastic cell lineage associated with human gastric adenocarcinoma. Lab.Invest. 1999;79:639–646. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

