The loss of Nf1 transiently promotes self-renewal but not tumorigenesis by neural crest stem cells
- PMID: 18242513
- PMCID: PMC2566828
- DOI: 10.1016/j.ccr.2008.01.003
The loss of Nf1 transiently promotes self-renewal but not tumorigenesis by neural crest stem cells
Abstract
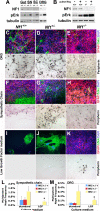
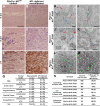
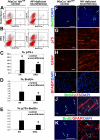
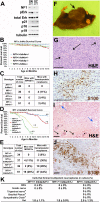
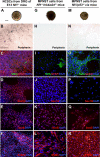
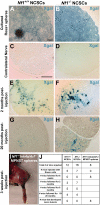
Neurofibromatosis is caused by the loss of neurofibromin (Nf1), leading to peripheral nervous system (PNS) tumors, including neurofibromas and malignant peripheral nerve sheath tumors (MPNSTs). A long-standing question has been whether these tumors arise from neural crest stem cells (NCSCs) or differentiated glia. Germline or conditional Nf1 deficiency caused a transient increase in NCSC frequency and self-renewal in most regions of the fetal PNS. However, Nf1-deficient NCSCs did not persist postnatally in regions of the PNS that developed tumors and could not form tumors upon transplantation into adult nerves. Adult P0a-Cre+Nf1(fl/-) mice developed neurofibromas, and Nf1(+/-)Ink4a/Arf(-/-) and Nf1/p53(+/-) mice developed MPNSTs, but NCSCs did not persist postnatally in affected locations in these mice. Tumors appeared to arise from differentiated glia, not NCSCs.
Figures

References
-
- Agesen TH, Florenes VA, Molenaar WM, Lind GE, Berner JM, Plaat BE, Komdeur R, Myklebost O, van den Berg E, Lothe RA. Expression patterns of cell cycle components in sporadic and neurofibromatosis type 1-related malignant peripheral nerve sheath tumors. J Neuropathol Exp Neurol. 2005;64:74–81. - PubMed
-
- Bixby S, Kruger GM, Mosher JT, Joseph NM, Morrison SJ. Cell-intrinsic differences between stem cells from different regions of the peripheral nervous system regulate the generation of neural diversity. Neuron. 2002;35:643–656. - PubMed
-
- Brannan CI, Perkins AS, Vogel KS, Ratner N, Nordlund ML, Reid SW, Buchberg AM, Jenkins NA, Parada LF, Copeland NG. Targeted disruption of the neurofibromatosis type-1 gene leads to developmental abnormalities in heart and various neural crest-derived tissues. Genes and Development. 1994;8:1019–1029. - PubMed
-
- Cichowski K, Shih TS, Schmitt E, Santiago S, Reilly K, McLaughlin ME, Bronson RT, Jacks T. Mouse models of tumor development in neurofibromatosis type 1. Science. 1999;286:2172–2176. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

