AID-dependent activation of a MYC transgene induces multiple myeloma in a conditional mouse model of post-germinal center malignancies
- PMID: 18242516
- PMCID: PMC2255064
- DOI: 10.1016/j.ccr.2008.01.007
AID-dependent activation of a MYC transgene induces multiple myeloma in a conditional mouse model of post-germinal center malignancies
Abstract
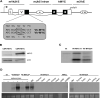
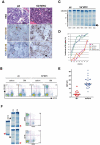
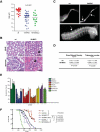
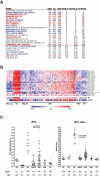
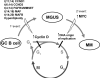
By misdirecting the activity of Activation-Induced Deaminase (AID) to a conditional MYC transgene, we have achieved sporadic, AID-dependent MYC activation in germinal center B cells of Vk*MYC mice. Whereas control C57BL/6 mice develop benign monoclonal gammopathy with age, all Vk*MYC mice progress to an indolent multiple myeloma associated with the biological and clinical features highly characteristic of the human disease. Furthermore, antigen-dependent myeloma could be induced by immunization with a T-dependent antigen. Consistent with these findings in mice, more frequent MYC rearrangements, elevated levels of MYC mRNA, and MYC target genes distinguish human patients with multiple myeloma from individuals with monoclonal gammopathy, implicating a causal role for MYC in the progression of monoclonal gammopathy to multiple myeloma.
Figures


References
-
- Adams JM, Harris AW, Pinkert CA, Corcoran LM, Alexander WS, Cory S, Palmiter RD, Brinster RL. The c-myc oncogene driven by immunoglobulin enhancers induces lymphoid malignancy in transgenic mice. Nature. 1985;318:533–538. - PubMed
-
- Alexanian R, Barlogie B, Dixon D. High-dose glucocorticoid treatment of resistant myeloma. Ann Intern Med. 1986;105:8–11. - PubMed
-
- Allen SL, Coleman M. Aggressive phase multiple myeloma: a terminal anaplastic transformation resembling high-grade lymphoma. Cancer Invest. 1990;8:417–424. - PubMed
-
- Avet-Loiseau H, Attal M, Moreau P, Charbonnel C, Garban F, Hulin C, Leyvraz S, Michallet M, Yakoub-Agha I, Garderet L, et al. Genetic abnormalities and survival in multiple myeloma: the experience of the Intergroupe Francophone du Myelome. Blood. 2007;109:3489–3495. - PubMed
-
- Avet-Loiseau H, Gerson F, Magrangeas F, Minvielle S, Harousseau JL, Bataille R. Rearrangements of the c-myc oncogene are present in 15% of primary human multiple myeloma tumors. Blood. 2001;98:3082–3086. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

