Acetylation of prostaglandin H2 synthases by aspirin is inhibited by redox cycling of the peroxidase
- PMID: 18242581
- PMCID: PMC2693035
- DOI: 10.1016/j.bcp.2007.12.005
Acetylation of prostaglandin H2 synthases by aspirin is inhibited by redox cycling of the peroxidase
Abstract
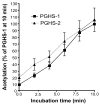
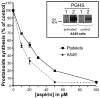
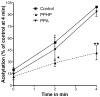
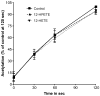
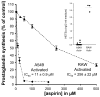
Aspirin exerts its unique pharmacological effects by irreversibly acetylating a serine residue in the cyclooxygenase site of prostaglandin-H2-synthases (PGHSs). Despite the irreversibility of the inhibition, the potency of aspirin varies remarkably between cell types, suggesting that molecular determinants could contribute to cellular selectivity. Using purified enzymes, we found no evidence that aspirin is selective for either of the two PGHS isoforms, and we showed that hydroperoxide substrates of the PGHS peroxidase inhibited the rate of acetylation of PGHS-1 by 68%. Using PGHS-1 reconstituted with cobalt protoporphyrin, a heme devoid of peroxidase activity, we demonstrated that reversal by hydroperoxides of the aspirin-mediated acetylation depends upon the catalytic activity of the PGHS peroxidase. We demonstrated that inhibition of PGHS-2 by aspirin in cells in culture is reversed by 12-hydroperoxyeicosatetraenoic acid dose-dependently (ED50=0.58+/-0.15 microM) and that in cells with high levels of hydroperoxy-fatty acids (RAW264.7) the efficacy of aspirin is markedly decreased as compared to cells with low levels of hydroperoxides (A549; IC50s=256+/-22 microM and 11.0+/-0.9 microM, respectively). Together, these findings indicate that acetylation of the PGHSs by aspirin is regulated by the catalytic activity of the peroxidase, which yields a higher oxidative state of the enzyme.
Figures
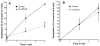
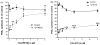
References
-
- Smith WL, Lands WE. Stimulation and blockade of prostaglandin biosynthesis. J Biol Chem. 1971;246:6700–2. - PubMed
-
- Lecomte M, Laneuville O, Ji C, DeWitt DL, Smith WL. Acetylation of human prostaglandin endoperoxide synthase-2 (cyclooxygenase-2) by aspirin. J Biol Chem. 1994;269:13207–15. - PubMed
-
- Roth GJ, Machuga ET, Ozols J. Isolation and covalent structure of the aspirin-modified, active-site region of prostaglandin synthetase. Biochemistry. 1983;22:4672–5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

