Peripheral inflammation is associated with altered substantia nigra activity and psychomotor slowing in humans
- PMID: 18242584
- PMCID: PMC2885493
- DOI: 10.1016/j.biopsych.2007.12.007
Peripheral inflammation is associated with altered substantia nigra activity and psychomotor slowing in humans
Abstract
Background: Systemic infections commonly cause sickness symptoms including psychomotor retardation. Inflammatory cytokines released during the innate immune response are implicated in the communication of peripheral inflammatory signals to the brain.
Methods: We used functional magnetic resonance brain imaging (fMRI) to investigate neural effects of peripheral inflammation following typhoid vaccination in 16 healthy men, using a double-blind, randomized, crossover-controlled design.
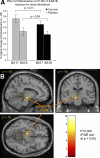
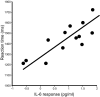
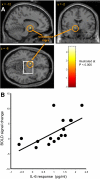
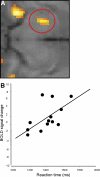
Results: Vaccination had no global effect on neurovascular coupling but markedly perturbed neural reactivity within substantia nigra during low-level visual stimulation. During a cognitive task, individuals in whom typhoid vaccination engendered higher levels of circulating interleukin-6 had significantly slower reaction time responses. Prolonged reaction times and larger interleukin-6 responses were associated with evoked neural activity within substantia nigra.
Conclusions: Our findings provide mechanistic insights into the interaction between inflammation and neurocognitive performance, specifically implicating circulating cytokines and midbrain dopaminergic nuclei in mediating the psychomotor consequences of systemic infection.
Figures



References
-
- Dantzer R., Bluthe R.M., Castanon N., Kelly K.W., Konsman J.-P., Laye S. Cytokines, sickness behavior, and depression. In: Ader R., editor. Psychoneuroimmunology. 4th ed. Elsevier; New York: 2007. pp. 281–318.
-
- Hart B.L. Biological basis of the behavior of sick animals. Neurosci Biobehav Rev. 1988;12:123–137. - PubMed
-
- Capuron L., Miller A.H. Cytokines and psychopathology: Lessons from interferon-alpha. Biol Psychiatry. 2004;56:819–824. - PubMed
-
- Wright C.E., Strike P.C., Brydon L., Steptoe A. Acute inflammation and negative mood: Mediation by cytokine activation. Brain Behav Immun. 2005;19:345–350. - PubMed
-
- Krabbe K.S., Reichenberg A., Yirmiya R., Smed A., Pedersen B.K., Bruunsgaard H. Low-dose endotoxemia and human neuropsychological functions. Brain Behav Immun. 2005;19:453–460. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

