The in-depth evaluation of suspected mitochondrial disease
- PMID: 18243024
- PMCID: PMC2810849
- DOI: 10.1016/j.ymgme.2007.11.018
The in-depth evaluation of suspected mitochondrial disease
Abstract
Mitochondrial disease confirmation and establishment of a specific molecular diagnosis requires extensive clinical and laboratory evaluation. Dual genome origins of mitochondrial disease, multi-organ system manifestations, and an ever increasing spectrum of recognized phenotypes represent the main diagnostic challenges. To overcome these obstacles, compiling information from a variety of diagnostic laboratory modalities can often provide sufficient evidence to establish an etiology. These include blood and tissue histochemical and analyte measurements, neuroimaging, provocative testing, enzymatic assays of tissue samples and cultured cells, as well as DNA analysis. As interpretation of results from these multifaceted investigations can become quite complex, the Diagnostic Committee of the Mitochondrial Medicine Society developed this review to provide an overview of currently available and emerging methodologies for the diagnosis of primary mitochondrial disease, with a focus on disorders characterized by impairment of oxidative phosphorylation. The aim of this work is to facilitate the diagnosis of mitochondrial disease by geneticists, neurologists, and other metabolic specialists who face the challenge of evaluating patients of all ages with suspected mitochondrial disease.
Figures





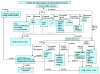
References
-
- Haas RH, P S, Falk MJ, Saneto RP, Wolf NI, Darin N, Cohen BH. Mitochondrial Disease: A practical approach for primary care physicians. Pediatrics. 2007 in press. - PubMed
-
- Bernier FP, et al. Diagnostic criteria for respiratory chain disorders in adults and children. Neurology. 2002;59(9):1406–11. - PubMed
-
- Wolf NI, Smeitink JA. Mitochondrial disorders: a proposal for consensus diagnostic criteria in infants and children. Neurology. 2002;59(9):1402–5. - PubMed
-
- DiMauro S, Hirano M. Mitochondrial encephalomyopathies: an update. Neuromuscul Disord. 2005;15(4):276–86. - PubMed
-
- Gellerich FN, et al. The problem of interlab variation in methods for mitochondrial disease diagnosis: enzymatic measurement of respiratory chain complexes. Mitochondrion. 2004;4(56):427–39. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

