NFATc1 balances quiescence and proliferation of skin stem cells
- PMID: 18243104
- PMCID: PMC2546702
- DOI: 10.1016/j.cell.2007.11.047
NFATc1 balances quiescence and proliferation of skin stem cells
Abstract
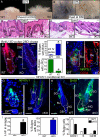
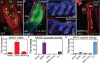
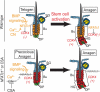
Quiescent adult stem cells reside in specialized niches where they become activated to proliferate and differentiate during tissue homeostasis and injury. How stem cell quiescence is governed is poorly understood. We report here that NFATc1 is preferentially expressed by hair follicle stem cells in their niche, where its expression is activated by BMP signaling upstream and it acts downstream to transcriptionally repress CDK4 and maintain stem cell quiescence. As stem cells become activated during hair growth, NFATc1 is downregulated, relieving CDK4 repression and activating proliferation. When calcineurin/NFATc1 signaling is suppressed, pharmacologically or via complete or conditional NFATc1 gene ablation, stem cells are activated prematurely, resulting in precocious follicular growth. Our findings may explain why patients receiving cyclosporine A for immunosuppressive therapy display excessive hair growth, and unveil a functional role for calcium-NFATc1-CDK4 circuitry in governing stem cell quiescence.
Figures


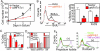

References
-
- Al-Daraji WI, Grant KR, Ryan K, Saxton A, Reynolds NJ. Localization of calcineurin/NFAT in human skin and psoriasis and inhibition of calcineurin/NFAT activation in human keratinocytes by cyclosporin A. J Invest Dermatol. 2002;118:779–788. - PubMed
-
- Andl T, Ahn K, Kairo A, Chu EY, Wine-Lee L, Reddy ST, Croft NJ, Cebra-Thomas JA, Metzger D, Chambon P, et al. Epithelial Bmpr1a regulates differentiation and proliferation in postnatal hair follicles and is essential for tooth development. Development. 2004;131:2257–2268. - PubMed
-
- Andl T, Reddy ST, Gaddapara T, Millar SE. WNT signals are required for the initiation of hair follicle development. Dev Cell. 2002;2:643–653. - PubMed
-
- Aramburu J, Garcia-Cozar F, Raghavan A, Okamura H, Rao A, Hogan PG. Selective inhibition of NFAT activation by a peptide spanning the calcineurin targeting site of NFAT. Mol Cell. 1998;1:627–637. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

