An HSV-1 gD mutant virus as an entry-impaired live virus vaccine
- PMID: 18243431
- PMCID: PMC2680698
- DOI: 10.1016/j.vaccine.2007.12.032
An HSV-1 gD mutant virus as an entry-impaired live virus vaccine
Abstract
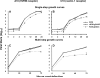
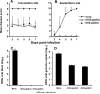
HSV-1 glycoprotein D (gD) interacts with HVEM and nectin-1 cell receptors to initiate virus entry. We prepared an HSV-1 strain with mutations in the gD gene at amino acid residues 3 and 38 by changing alanine to cysteine and tyrosine to cysteine, respectively (A3C/Y38C). These mutations were constructed with the intent of evaluating infection in vivo when virus enters by HVEM but not nectin-1 receptors and were based on prior reports demonstrating that purified gDA3C/Y38C protein binds to HVEM but not to nectin-1. While preparing a high-titered purified virus pool, the cysteine mutation at position 38 reverted to tyrosine, which occurred on two separate occasions. The resultant HSV-1 strain, KOS-gDA3C, had a single amino acid mutation at residue 3 and exhibited reduced entry into both HVEM and nectin-1 expressing cells. When tested in the murine flank model, the mutant virus was markedly attenuated for virulence and caused only mild disease, while the parental and rescued viruses produced much more severe disease. Thirty days after KOS-gDA3C infection, mice were challenged with a lethal dose of HSV-1 and were highly resistant to disease. The KOS-gDA3C mutation was stable during 30 passages in vitro and was present in each of 3 isolates obtained from infected mice. Therefore, this gD mutant virus impaired in entry may represent a novel candidate for an attenuated live HSV-1 vaccine.
Figures

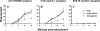

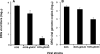
References
-
- Smith JS, Robinson NJ. Age specific prevalence of infection with herpes simplex virus type 2 and 1: a global review. Journal of Infectious Diseases. 2002;186:S3–28. - PubMed
-
- Xu Y, Sternberg MR, Kottiri BJ, McQuillan GM, Lee FK, Nehmias AJ, Barman SM, Markowitz LE. Trends in herpes simplex type 1 and type 2 seroprevalence in the United States. JAMA. 2006;296:964–73. - PubMed
-
- Whitley Rj, Lakeman F. Herpes virus infections of central nervous system: therapeutic and diagnostic considerations. Clinical Infectious Diseases. 1995;20:414–20. - PubMed
-
- Raschilas FWM, Delatour F, Chaffaut C, De Broucker T, Chevret S, Lebon P, Canton P, Rozenberg F. Outcome of and prognostic factors for herpes simplex encephalitis in adult patients: results of a multicenter study. Clinical Infectious Diseases. 2002;35:254–60. - PubMed
-
- Liesegang TJ. Epidemology of ocular herpes simplex. Natural history in Rochester, Minn, 1950 through 1982. Archives of Ophthalmology. 1889;107:171–85. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

