A novel competitive repopulation strategy to quantitate engraftment of ex vivo manipulated murine marrow cells in submyeloablated hosts
- PMID: 18243491
- PMCID: PMC2373923
- DOI: 10.1016/j.exphem.2007.12.002
A novel competitive repopulation strategy to quantitate engraftment of ex vivo manipulated murine marrow cells in submyeloablated hosts
Abstract
Objective: Standard competitive repopulation assays have proven valuable in evaluating engraftment potential in ablated hosts, permitting comparisons between various test cell populations. However, no similar method exists to compare engraftment of test cells in submyeloablated hosts, which would be helpful given the applications of reduced-intensity conditioning for hematopoietic gene-replacement therapy and other cellular therapies. Here, we developed a novel assay to quantitate engraftment of hematopoietic stem cells in submyeloablated hosts.
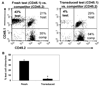
Materials and methods: Engraftment of murine marrow cells transduced with retroviral vectors using two separate protocols was compared to engraftment of fresh untreated competitor cells within low-dose radiation-conditioned hosts using a "three-way" marking system, so that test, competitor, and host cell chimerism could be reliably determined posttransplantation.
Results: We demonstrate that the repopulating ability of marrow cells transduced using two distinct protocols was reduced approximately 10-fold compared to fresh competitor cells in submyeloablated hosts utilizing the novel "three-way" transplant assay.
Conclusions: Murine marrow cells transduced using a clinically applicable protocol acquire an engraftment defect in submyeloablated hosts, similar to cells transduced using a research protocol. We conclude that the submyeloablative competitive repopulation assay described here will be of benefit to comparatively assess the engraftment ability of manipulated hematopoietic stem cells using various culture protocols, such as to test the impact of modifications in transduction protocols needed to attain therapeutic levels of gene-corrected blood cells, or the effect of ex vivo expansion protocols on engraftment potential.
Figures




References
-
- Voltarelli JC, Couri CE, Stracieri AB, et al. Autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabetes mellitus. JAMA. 2007;297:1568–1576. - PubMed
-
- Burt RK, Traynor A, Statkute L, et al. Nonmyeloablative hematopoietic stem cell transplantation for systemic lupus erythematosus. JAMA. 2006;295:527–535. - PubMed
-
- Khalil PN, Weiler V, Nelson PJ, et al. Nonmyeloablative stem cell therapy enhances microcirculation and tissue regeneration in murine inflammatory bowel disease. Gastroenterology. 2007;132:944–954. - PubMed
-
- Down J, Ploemacher R. Transient and permanent engraftment potential of murine hematopoietic stem cell subsets: differential effects of host conditioning with gamma radiation and cytotoxic drugs. Exp Hematol. 1993;21:913–921. - PubMed
-
- Tomita Y, Sachs D, Sykes M. Myelosuppressive conditioning is required to achieve engraftment of pluripotent stem cells contained in moderate doses of syngeneic bone marrow. Blood. 1994;83:939–948. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

