Mitochondrial import and accumulation of alpha-synuclein impair complex I in human dopaminergic neuronal cultures and Parkinson disease brain
- PMID: 18245082
- PMCID: PMC2431021
- DOI: 10.1074/jbc.M710012200
Mitochondrial import and accumulation of alpha-synuclein impair complex I in human dopaminergic neuronal cultures and Parkinson disease brain
Abstract
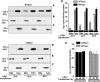
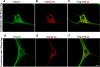
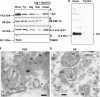
Alpha-synuclein, a protein implicated in the pathogenesis of Parkinson disease (PD), is thought to affect mitochondrial functions, although the mechanisms of its action remain unclear. In this study we show that the N-terminal 32 amino acids of human alpha-synuclein contain cryptic mitochondrial targeting signal, which is important for mitochondrial targeting of alpha-synuclein. Mitochondrial imported alpha-synuclein is predominantly associated with the inner membrane. Accumulation of wild-type alpha-synuclein in the mitochondria of human dopaminergic neurons caused reduced mitochondrial complex I activity and increased production of reactive oxygen species. However, these defects occurred at an early time point in dopaminergic neurons expressing familial alpha-synuclein with A53T mutation as compared with wild-type alpha-synuclein. Importantly, alpha-synuclein that lacks mitochondrial targeting signal failed to target to the mitochondria and showed no detectable effect on complex I function. The PD relevance of these results was investigated using mitochondria of substantia nigra, striatum, and cerebellum of postmortem late-onset PD and normal human brains. Results showed the constitutive presence of approximately 14-kDa alpha-synuclein in the mitochondria of all three brain regions of normal subjects. Mitochondria of PD-vulnerable substantia nigra and striatum but not cerebellum from PD subjects showed significant accumulation of alpha-synuclein and decreased complex I activity. Analysis of mitochondria from PD brain and alpha-synuclein expressing dopaminergic neuronal cultures using blue native gel electrophoresis and immunocapture technique showed the association of alpha-synuclein with complex I. These results provide evidence that mitochondrial accumulated alpha-synuclein may interact with complex I and interfere with its functions.
Figures





References
-
- Bennett, M. C. (2005) Pharmacol. Ther. 105 311-331 - PubMed
-
- Dawson, T. M., and Dawson, V. L. (2003) Science 302 819-822 - PubMed
-
- Abou-Sleiman, P. M., Muqit, M. M., McDonald, N. Q., Yang, Y. X., Gandhi, S., Healy, D. G., Harvey, K., Harvey, R. J., Deas, E., Bhatia, K., Quinn, N., Lees, A., Latchman, D. S., and Wood, N. W. (2006) Ann. Neurol. 60 414-419 - PubMed
-
- Fiskum, G., Starkov, A., Polster, B. M., and Chinopoulos, C. (2003) Ann. N. Y. Acad. Sci. 991 111-119 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

