The Saccharomyces cerevisiae homolog of p24 is essential for maintaining the association of p150Glued with the dynactin complex
- PMID: 18245366
- PMCID: PMC2248349
- DOI: 10.1534/genetics.107.079103
The Saccharomyces cerevisiae homolog of p24 is essential for maintaining the association of p150Glued with the dynactin complex
Abstract
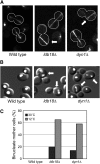
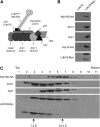
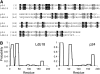
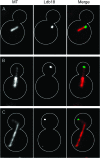
Stu1 is the Saccharomyces cerevisiae member of the CLASP family of microtubule plus-end tracking proteins and is essential for spindle formation. A genomewide screen for gene deletions that are lethal in combination with the temperature-sensitive stu1-5 allele identified ldb18Delta. ldb18Delta cells exhibit defects in spindle orientation similar to those caused by a block in the dynein pathway. Consistent with this observation, ldb18Delta is synthetic lethal with mutations affecting the Kar9 spindle orientation pathway, but not with those affecting the dynein pathway. We show that Ldb18 is a component of dynactin, a complex required for dynein activity in yeast and mammalian cells. Ldb18 shares modest sequence and structural homology with the mammalian dynactin component p24. It interacts with dynactin proteins in two-hybrid and co-immunoprecipitation assays, and comigrates with them as a 20 S complex during sucrose gradient sedimentation. In ldb18Delta cells, the interaction between Nip100 (p150(Glued)) and Jnm1 (dynamitin) is disrupted, while the interaction between Jnm1 and Arp1 is not affected. These results indicate that p24 is required for attachment of the p150(Glued) arm to dynamitin and the remainder of the dynactin complex. The genetic interaction of ldb18Delta with stu1-5 also supports the notion that dynein/dynactin helps to generate a spindle pole separating force.
Figures
References
-
- Brachmann, C. B., A. Davies, G. J. Cost, E. Caputo, J. Li et al., 1998. Designer deletion strains derived from Saccharomyces cerevisiae S288C: a useful set of strains and plasmids for PCR-mediated gene disruption and other applications. Yeast 14 115–132. - PubMed
-
- Corbacho, I., I. Olivero and L. M. Hernandez, 2005. A genome-wide screen for Saccharomyces cerevisiae nonessential genes involved in mannosyl phosphate transfer to mannoprotein-linked oligosaccharides. Fungal Genet. Biol. 42 773–790. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

