Mechanistic duality of transcription factor function in phytochrome signaling
- PMID: 18245378
- PMCID: PMC2538903
- DOI: 10.1073/pnas.0711675105
Mechanistic duality of transcription factor function in phytochrome signaling
Abstract
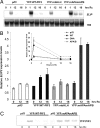
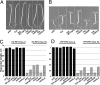
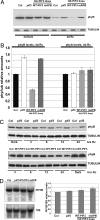
The phytochrome (phy) family of sensory photoreceptors (phyA-E in Arabidopsis) elicit changes in gene expression after light-induced migration to the nucleus, where they interact with basic helix-loop-helix transcription factors, such as phytochrome-interacting factor 3 (PIF3). The mechanism by which PIF3 relays phy signals, both early after initial light exposure and later during long-term irradiation, is not understood. Using transgenically expressed PIF3 variants, carrying site-specific amino acid substitutions that block the protein from binding either to DNA, phyA, and/or phyB, we examined the involvement of PIF3 in early, phy-induced marker gene expression and in modulating long-term, phy-imposed inhibition of hypocotyl cell elongation under prolonged, continuous irradiation. We describe an unanticipated dual mechanism of PIF3 action that involves the temporal uncoupling of its two most central molecular functions. We find that in early signaling, PIF3 acts positively as a transcription factor, exclusively requiring its DNA-binding capacity. Contrary to previous proposals, PIF3 functions as a constitutive coactivator in this process, without the need for phy binding and subsequent phy-induced modifications. This finding implies that another factor(s) is conditionally activated by phy and functions in concert with PIF3, to induce target gene transcription. In contrast, during long-term irradiations, PIF3 acts exclusively through its phyB-interacting capacity to control hypocotyl cell elongation, independently of its ability to bind DNA. Unexpectedly, PIF3 uses this capacity to regulate phyB protein abundance (and thereby global photosensory sensitivity) to modulate this long-term response rather than participating directly in the transduction chain as a signaling intermediate.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Schäfer E, Nagy F, editors. Photomorphogenesis in Plants and Bacteria. Dordrecht: Springer; 2006.
-
- Tu S-L, Lagarias CJ. In: Handbook of Photosensory Receptors. Briggs WR, Spudich JL, editors. Weinheim: Wiley; 2005. pp. 121–150.
-
- Quail PH. Phytochrome photosensory signalling networks. Nature Rev Mol Cell Biol. 2002;3:85–93. - PubMed
-
- Quail PH. Phytochrome-regulated gene expression. J Integrative Plant Biol. 2007;49:11–20.
-
- Jiao Y, Lau OS, Deng XW. Light-regulated transcriptional networks in higher plants. Nat Rev Genet. 2007;8:217–230. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

