A role for the receptor for advanced glycation end products in idiopathic pulmonary fibrosis
- PMID: 18245812
- PMCID: PMC2258251
- DOI: 10.2353/ajpath.2008.070569
A role for the receptor for advanced glycation end products in idiopathic pulmonary fibrosis
Abstract
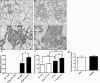
Idiopathic pulmonary fibrosis (IPF) is a severely debilitating disease associated with a dismal prognosis. There are currently no effective therapies for IPF, thus the identification of novel therapeutic targets is greatly needed. The receptor for advanced glycation end products (RAGE) is a member of the immunoglobulin superfamily of cell surface receptors whose activation has been linked to various pathologies. In healthy adult animals, RAGE is expressed at the highest levels in the lung compared to other tissues. To investigate the hypothesis that RAGE is involved in IPF pathogenesis, we have examined its expression in two mouse models of pulmonary fibrosis and in human tissue from IPF patients. In each instance we observed a depletion of membrane RAGE and its soluble (decoy) isoform, sRAGE, in fibrotic lungs. In contrast to other diseases in which RAGE signaling promotes pathology, immunohistochemical and hydroxyproline quantification studies on aged RAGE-null mice indicate that these mice spontaneously develop pulmonary fibrosis-like alterations. Furthermore, when subjected to a model of pulmonary fibrosis, RAGE-null mice developed more severe fibrosis, as measured by hydroxyproline assay and histological scoring, than wild-type controls. Combined with data from other studies on mouse models of pulmonary fibrosis and human IPF tissues indicate that loss of RAGE contributes to IPF pathogenesis.
Figures
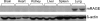
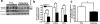
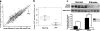

Similar articles
-
Paradoxical function for the receptor for advanced glycation end products in mouse models of pulmonary fibrosis.Int J Clin Exp Pathol. 2011 Mar;4(3):241-54. Epub 2011 Mar 21. Int J Clin Exp Pathol. 2011. PMID: 21487520 Free PMC article.
-
The role of the receptor for advanced glycation end-products in a murine model of silicosis.PLoS One. 2010 Mar 19;5(3):e9604. doi: 10.1371/journal.pone.0009604. PLoS One. 2010. PMID: 20333255 Free PMC article.
-
Advanced glycation end-products and receptor for advanced glycation end-products expression in patients with idiopathic pulmonary fibrosis and NSIP.Int J Clin Exp Pathol. 2013 Dec 15;7(1):221-8. eCollection 2014. Int J Clin Exp Pathol. 2013. PMID: 24427342 Free PMC article.
-
Association of the RAGE/RAGE-ligand axis with interstitial lung disease and its acute exacerbation.Respir Investig. 2022 Jul;60(4):531-542. doi: 10.1016/j.resinv.2022.04.004. Epub 2022 May 2. Respir Investig. 2022. PMID: 35504814 Review.
-
The receptor for advanced glycation end products (RAGE) and the lung.J Biomed Biotechnol. 2010;2010:917108. doi: 10.1155/2010/917108. Epub 2010 Jan 19. J Biomed Biotechnol. 2010. PMID: 20145712 Free PMC article. Review.
Cited by
-
S100A12 and the Airway Smooth Muscle: Beyond Inflammation and Constriction.J Allergy Ther. 2012 Apr 20;3(Suppl 1):S1-007. doi: 10.4172/2155-6121.S1-007. J Allergy Ther. 2012. PMID: 25984393 Free PMC article.
-
Impact of Transcriptomics on Our Understanding of Pulmonary Fibrosis.Front Med (Lausanne). 2018 Apr 4;5:87. doi: 10.3389/fmed.2018.00087. eCollection 2018. Front Med (Lausanne). 2018. PMID: 29670881 Free PMC article. Review.
-
Receptor for advanced glycation end products and its involvement in inflammatory diseases.Int J Inflam. 2013;2013:403460. doi: 10.1155/2013/403460. Epub 2013 Sep 11. Int J Inflam. 2013. PMID: 24102034 Free PMC article. Review.
-
Wnt/beta-catenin signaling promotes renal interstitial fibrosis.J Am Soc Nephrol. 2009 Apr;20(4):765-76. doi: 10.1681/ASN.2008060566. Epub 2009 Mar 18. J Am Soc Nephrol. 2009. PMID: 19297557 Free PMC article.
-
Pharmacological targets of SGLT2 inhibitors on IgA nephropathy and membranous nephropathy: a mendelian randomization study.Front Pharmacol. 2024 May 22;15:1399881. doi: 10.3389/fphar.2024.1399881. eCollection 2024. Front Pharmacol. 2024. PMID: 38846092 Free PMC article.
References
-
- Crystal RG, Bitterman PB, Rennard SI, Hance AJ, Keogh BA. Interstitial lung diseases of unknown cause. Disorders characterized by chronic inflammation of the lower respiratory tract. N Engl J Med. 1984;310:235–244. - PubMed
-
- Collard HR, King TE., Jr Demystifying idiopathic interstitial pneumonia. Arch Intern Med. 2003;163:17–29. - PubMed
-
- Selman M, King TE, Pardo A. Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann Intern Med. 2001;134:136–151. - PubMed
-
- Schmidt AM, Vianna M, Gerlach M, Brett J, Ryan J, Kao J, Esposito C, Hegarty H, Hurley W, Clauss M, Wang F, Pan Y-CE, Tsang TC, Stern D. Isolation and characterization of two binding proteins for advanced glycosylation end products from bovine lung which are present on the endothelial cell surface. J Biol Chem. 1992;267:14987–14997. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

