Glucocorticoids and cyclic AMP selectively increase hepatic lipin-1 expression, and insulin acts antagonistically
- PMID: 18245816
- PMCID: PMC2311443
- DOI: 10.1194/jlr.M800013-JLR200
Glucocorticoids and cyclic AMP selectively increase hepatic lipin-1 expression, and insulin acts antagonistically
Abstract
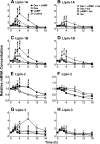
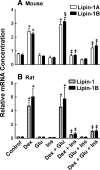
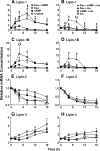
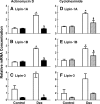
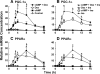
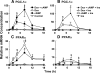
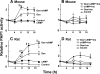
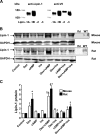
Glucocorticoids (GCs) increase hepatic phosphatidate phosphatase (PAP1) activity. This is important in enhancing the liver's capacity for storing fatty acids as triacylglycerols (TAGs) that can be used subsequently for beta-oxidation or VLDL secretion. PAP1 catalyzes the conversion of phosphatidate to diacylglycerol, a key substrate for TAG and phospholipid biosynthesis. PAP1 enzymes in liver include lipin-1A and -1B (alternatively spliced isoforms) and two distinct gene products, lipin-2 and lipin-3. We determined the mechanisms by which the composite PAP1 activity is regulated using rat and mouse hepatocytes. Levels of lipin-1A and -1B mRNA were increased by dexamethasone (dex; a synthetic GC), and this resulted in increased lipin-1 synthesis, protein levels, and PAP1 activity. The stimulatory effect of dex on lipin-1 expression was enhanced by glucagon or cAMP and antagonized by insulin. Lipin-2 and lipin-3 mRNA were not increased by dex/cAMP, indicating that increased PAP1 activity is attributable specifically to enhanced lipin-1 expression. This work provides the first evidence for the differential regulation of lipin activities. Selective lipin-1 expression explains the GC and cAMP effects on increased hepatic PAP1 activity, which occurs in hepatic steatosis during starvation, diabetes, stress, and ethanol consumption.
Figures
Similar articles
-
Three mammalian lipins act as phosphatidate phosphatases with distinct tissue expression patterns.J Biol Chem. 2007 Feb 9;282(6):3450-7. doi: 10.1074/jbc.M610745200. Epub 2006 Dec 7. J Biol Chem. 2007. PMID: 17158099
-
The level and compartmentalization of phosphatidate phosphatase-1 (lipin-1) control the assembly and secretion of hepatic VLDL.J Lipid Res. 2009 Jan;50(1):47-58. doi: 10.1194/jlr.M800204-JLR200. Epub 2008 Sep 3. J Lipid Res. 2009. PMID: 18769019
-
Thematic Review Series: Glycerolipids. Multiple roles for lipins/phosphatidate phosphatase enzymes in lipid metabolism.J Lipid Res. 2008 Dec;49(12):2493-503. doi: 10.1194/jlr.R800019-JLR200. Epub 2008 Sep 12. J Lipid Res. 2008. PMID: 18791037 Free PMC article. Review.
-
Combination of lipid metabolism alterations and their sensitivity to inflammatory cytokines in human lipin-1-deficient myoblasts.Biochim Biophys Acta. 2013 Dec;1832(12):2103-14. doi: 10.1016/j.bbadis.2013.07.021. Epub 2013 Aug 6. Biochim Biophys Acta. 2013. PMID: 23928362 Free PMC article.
-
Lipin - The bridge between hepatic glycerolipid biosynthesis and lipoprotein metabolism.Biochim Biophys Acta. 2010 Dec;1801(12):1249-59. doi: 10.1016/j.bbalip.2010.07.008. Epub 2010 Aug 6. Biochim Biophys Acta. 2010. PMID: 20692363 Review.
Cited by
-
A conserved serine residue is required for the phosphatidate phosphatase activity but not the transcriptional coactivator functions of lipin-1 and lipin-2.J Biol Chem. 2009 Oct 23;284(43):29968-78. doi: 10.1074/jbc.M109.023663. Epub 2009 Aug 28. J Biol Chem. 2009. PMID: 19717560 Free PMC article.
-
Lipin 2/3 phosphatidic acid phosphatases maintain phospholipid homeostasis to regulate chylomicron synthesis.J Clin Invest. 2019 Jan 2;129(1):281-295. doi: 10.1172/JCI122595. Epub 2018 Dec 3. J Clin Invest. 2019. PMID: 30507612 Free PMC article.
-
Regulation of hepatic lipin-1 by ethanol: role of AMP-activated protein kinase/sterol regulatory element-binding protein 1 signaling in mice.Hepatology. 2012 Feb;55(2):437-46. doi: 10.1002/hep.24708. Epub 2011 Dec 29. Hepatology. 2012. PMID: 21953514 Free PMC article.
-
Lipin-1, a Versatile Regulator of Lipid Homeostasis, Is a Potential Target for Fighting Cancer.Int J Mol Sci. 2021 Apr 23;22(9):4419. doi: 10.3390/ijms22094419. Int J Mol Sci. 2021. PMID: 33922580 Free PMC article. Review.
-
Regulation of triglyceride metabolism by glucocorticoid receptor.Cell Biosci. 2012 May 28;2(1):19. doi: 10.1186/2045-3701-2-19. Cell Biosci. 2012. PMID: 22640645 Free PMC article.
References
-
- Jamal Z., A. Martin, A. Gomez-Munoz, and D. N. Brindley. 1991. Plasma membrane fractions from rat liver contain a phosphatidate phosphohydrolase distinct from that in the endoplasmic reticulum and cytosol. J. Biol. Chem. 266 2988–2996. - PubMed
-
- Brindley D. N. 2004. Lipid phosphate phosphatases and related proteins: signaling functions in development, cell division, and cancer. J. Cell. Biochem. 92 900–912. - PubMed
-
- Donkor J., M. Sariahmetoglu, J. Dewald, D. N. Brindley, and K. Reue. 2007. Three mammalian lipins act as phosphatidate phosphatases with distinct tissue expression patterns. J. Biol. Chem. 282 3450–3457. - PubMed
-
- Brindley, D. N. 1988. Phosphatidate Phosphohydrolase: Its Role in Glycerolipid Synthesis. CRC Press, Boca Raton, FL. 21–77.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

