Human C-reactive protein promotes oxidized low density lipoprotein uptake and matrix metalloproteinase-9 release in Wistar rats
- PMID: 18245817
- PMCID: PMC2311439
- DOI: 10.1194/jlr.M700535-JLR200
Human C-reactive protein promotes oxidized low density lipoprotein uptake and matrix metalloproteinase-9 release in Wistar rats
Abstract
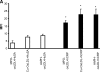
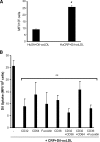
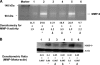
C-reactive protein (CRP) is present in the atherosclerotic plaques and appears to promote atherogenesis. Intraplaque CRP colocalizes with oxidized low density lipoprotein (OxLDL) and macrophages in human atherosclerotic lesions. Matrix metalloproteinase-9 (MMP-9) has been implicated in plaque rupture. CRP promotes OxLDL uptake and MMP induction in vitro; however, these have not been investigated in vivo. We examined the effect of CRP on OxLDL uptake and MMP-9 production in vivo in Wistar rats. CRP significantly increased OxLDL uptake in the peritoneal and sterile pouch macrophages compared with human serum albumin (huSA). CRP also significantly increased intracellular cholesteryl ester accumulation compared with huSA. The increased uptake of OxLDL by CRP was inhibited by pretreatment with antibodies to CD32, CD64, CD36, and fucoidin, suggesting uptake by both scavenger receptors and Fc-gamma receptors. Furthermore, CRP treatment increased MMP-9 activity in macrophages compared with huSA, which was abrogated by inhibitors to p38 mitogen-activated protein kinase, extracellular signal-regulated kinase (ERK), and nuclear factor (NF)-kappaB but not Jun N-terminal kinase (JNK) before human CRP treatment. Because OxLDL uptake by macrophages contributes to foam cell formation and MMP release contributes to plaque instability, this study provides novel in vivo evidence for the role of CRP in atherosclerosis.
Figures



Similar articles
-
C-reactive protein stimulates superoxide anion release and tissue factor activity in vivo.Atherosclerosis. 2009 Mar;203(1):67-74. doi: 10.1016/j.atherosclerosis.2008.05.060. Epub 2008 Jun 13. Atherosclerosis. 2009. PMID: 18621373 Free PMC article.
-
MMP-9 inhibition by ACE inhibitor reduces oxidized LDL-mediated foam-cell formation.J Atheroscler Thromb. 2010 Feb;17(1):97-105. doi: 10.5551/jat.1685. Epub 2010 Jan 21. J Atheroscler Thromb. 2010. PMID: 20093780
-
Ginkgo biloba extract inhibits oxidized low-density lipoprotein (oxLDL)-induced matrix metalloproteinase activation by the modulation of the lectin-like oxLDL receptor 1-regulated signaling pathway in human umbilical vein endothelial cells.J Vasc Surg. 2016 Jan;63(1):204-15.e1. doi: 10.1016/j.jvs.2014.05.098. Epub 2014 Jul 28. J Vasc Surg. 2016. PMID: 25080882
-
Postprandial lipoproteins and the molecular regulation of vascular homeostasis.Prog Lipid Res. 2013 Oct;52(4):446-64. doi: 10.1016/j.plipres.2013.06.001. Epub 2013 Jun 15. Prog Lipid Res. 2013. PMID: 23774609 Review.
-
Interrelatedness between C-reactive protein and oxidized low-density lipoprotein.Clin Chem Lab Med. 2015 Jan;53(1):29-34. doi: 10.1515/cclm-2014-0590. Clin Chem Lab Med. 2015. PMID: 25010779 Review.
Cited by
-
C-Reactive Protein: An In-Depth Look into Structure, Function, and Regulation.Int Sch Res Notices. 2014 Dec 15;2014:653045. doi: 10.1155/2014/653045. eCollection 2014. Int Sch Res Notices. 2014. PMID: 27433484 Free PMC article. Review.
-
Endogenous sex hormones and their associations with cardiovascular risk factors in post-menopausal women.J Endocrinol Invest. 2013 Sep;36(8):588-92. doi: 10.3275/8881. Epub 2013 Feb 27. J Endocrinol Invest. 2013. PMID: 23448998
-
Does C-reactive protein contribute to atherothrombosis via oxidant-mediated release of pro-thrombotic factors and activation of platelets?Front Physiol. 2012 Nov 16;3:433. doi: 10.3389/fphys.2012.00433. eCollection 2012. Front Physiol. 2012. PMID: 23162475 Free PMC article.
-
The pro-atherogenic effects of macrophages are reduced upon formation of a complex between C-reactive protein and lysophosphatidylcholine.J Inflamm (Lond). 2012 Oct 31;9(1):42. doi: 10.1186/1476-9255-9-42. J Inflamm (Lond). 2012. PMID: 23114023 Free PMC article.
-
Role of C-reactive protein in contributing to increased cardiovascular risk in metabolic syndrome.Curr Atheroscler Rep. 2010 Mar;12(2):110-8. doi: 10.1007/s11883-010-0098-3. Curr Atheroscler Rep. 2010. PMID: 20425246 Free PMC article. Review.
References
-
- Croce K., and P. Libby. 2007. Intertwining of thrombosis and inflammation in atherosclerosis. Curr. Opin. Hematol. 14 55–61. - PubMed
-
- Jialal I., S. Devaraj, and S. K. Venugopal. 2004. C-reactive protein: risk marker or mediator in atherothrombosis? Hypertension. 44 6–11. - PubMed
-
- Verma S., S. Devaraj, and I. Jialal. 2006. Is C-reactive protein an innocent bystander or proatherogenic culprit? C-reactive protein promotes atherothrombosis. Circulation. 113 2135–2150. - PubMed
-
- Ishikawa T., K. Hatakeyama, T. Imamura, Y. Shibata, Y. Hikichi, Y. Asada, and T. Eto. 2003. Involvement of CRP obtained by directional coronary atherectomy in plaque instability and developing restenosis in patients with stable or unstable angina pectoris. Am. J. Cardiol. 91 287–292. - PubMed
-
- Ballou C. P., and G. Lozanski. 1992. Induction of inflammatory cytokine release from cultured human monocytes by CRP. Cytokine. 4 361–368. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

