The impact of dissociation on transposon-mediated disease control strategies
- PMID: 18245837
- PMCID: PMC2278053
- DOI: 10.1534/genetics.107.082099
The impact of dissociation on transposon-mediated disease control strategies
Abstract
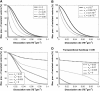
Vector-borne diseases such as malaria and dengue fever continue to be a major health concern through much of the world. The emergence of chloroquine-resistant strains of malaria and insecticide-resistant mosquitoes emphasize the need for novel methods of disease control. Recently, there has been much interest in the use of transposable elements to drive resistance genes into vector populations as a means of disease control. One concern that must be addressed before a release is performed is the potential loss of linkage between a transposable element and a resistance gene. Transposable elements such as P and hobo have been shown to produce internal deletion derivatives at a significant rate, and there is concern that a similar process could lead to loss of the resistance gene from the drive system following a transgenic release. Additionally, transposable elements such as Himar1 have been shown to transpose significantly more frequently when free of exogenous DNA. Here, we show that any transposon-mediated gene drive strategy must have an exceptionally low rate of dissociation if it is to be effective. Additionally, the resistance gene must confer a large selective advantage to the vector to surmount the effects of a moderate dissociation rate and transpositional handicap.
Figures



Similar articles
-
Germline transformation of Aedes fluviatilis (Diptera:Culicidae) with the piggyBac transposable element.Mem Inst Oswaldo Cruz. 2006 Nov;101(7):755-7. doi: 10.1590/s0074-02762006000700008. Mem Inst Oswaldo Cruz. 2006. PMID: 17160283
-
Mobility of the piggyBac transposon in embryos of the vectors of Dengue fever (Aedes albopictus) and La Crosse encephalitis (Ae. triseriatus).Mol Genet Genomics. 2001 Mar;265(1):66-71. doi: 10.1007/s004380000388. Mol Genet Genomics. 2001. PMID: 11370874
-
The effect of gene drive on containment of transgenic mosquitoes.J Theor Biol. 2009 May 21;258(2):250-65. doi: 10.1016/j.jtbi.2009.01.031. Epub 2009 Feb 7. J Theor Biol. 2009. PMID: 19490857
-
Gene vector and transposable element behavior in mosquitoes.J Exp Biol. 2003 Nov;206(Pt 21):3823-34. doi: 10.1242/jeb.00638. J Exp Biol. 2003. PMID: 14506218 Review.
-
Reflections on the Anopheles gambiae genome sequence, transgenic mosquitoes and the prospect for controlling malaria and other vector borne diseases.J Med Entomol. 2003 Sep;40(5):597-606. doi: 10.1603/0022-2585-40.5.597. J Med Entomol. 2003. PMID: 14596272 Review.
Cited by
-
Confinement of gene drive systems to local populations: a comparative analysis.J Theor Biol. 2012 Feb 7;294:153-71. doi: 10.1016/j.jtbi.2011.10.032. Epub 2011 Nov 9. J Theor Biol. 2012. PMID: 22094363 Free PMC article.
-
Engineering the genomes of wild insect populations: challenges, and opportunities provided by synthetic Medea selfish genetic elements.J Insect Physiol. 2010 Oct;56(10):1402-13. doi: 10.1016/j.jinsphys.2010.05.022. Epub 2010 Jun 9. J Insect Physiol. 2010. PMID: 20570677 Free PMC article. Review.
-
Evaluating paratransgenesis as a potential control strategy for African trypanosomiasis.PLoS Negl Trop Dis. 2013 Aug 15;7(8):e2374. doi: 10.1371/journal.pntd.0002374. eCollection 2013. PLoS Negl Trop Dis. 2013. PMID: 23967363 Free PMC article.
-
Conditions for success of engineered underdominance gene drive systems.J Theor Biol. 2017 Oct 7;430:128-140. doi: 10.1016/j.jtbi.2017.07.014. Epub 2017 Jul 17. J Theor Biol. 2017. PMID: 28728996 Free PMC article.
-
The cost-effectiveness of controlling dengue in Indonesia using wMel Wolbachia released at scale: a modelling study.BMC Med. 2020 Jul 9;18(1):186. doi: 10.1186/s12916-020-01638-2. BMC Med. 2020. PMID: 32641039 Free PMC article.
References
-
- Appawu, M. A., K. M. Bosompem, S. Dadzie, U. S. McKakpo, I. Anim-Baidoo et al., 2003. Detection of malaria sporozoites by standard ELISA and VecTest dipstick assay in field-collected anopheline mosquitoes from a malaria endemic site in Ghana. Trop. Med. Int. Health 8 1012–1017. - PubMed
-
- Ahmed, A. M., S. L. Baggott, R. Maingon and H. Hurd, 2002. The costs of mounting an immune response are reflected in the reproductive fitness of the mosquito Anopheles gambiae. Oikos 97 371–377.
-
- Beier, J. C., G. F. Killeen and J. I. Githure, 1999. Short report: entomologic inoculation rates and Plasmodium falciparum malaria prevalence in Africa. Am. J. Trop. Med. Hyg. 61 109–113. - PubMed
-
- Boëte, C., and J. C. Koella, 2003. A model for coevolution of immunity and immune evasion in vector-borne diseases with implications for the epidemiology of malaria. Am. Nat. 161 698–707. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

