Functioning of the Drosophila Wilms'-tumor-1-associated protein homolog, Fl(2)d, in Sex-lethal-dependent alternative splicing
- PMID: 18245840
- PMCID: PMC2248336
- DOI: 10.1534/genetics.107.081679
Functioning of the Drosophila Wilms'-tumor-1-associated protein homolog, Fl(2)d, in Sex-lethal-dependent alternative splicing
Abstract
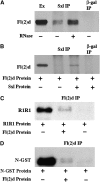
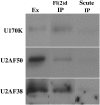
fl(2)d, the Drosophila homolog of Wilms'-tumor-1-associated protein (WTAP), regulates the alternative splicing of Sex-lethal (Sxl), transformer (tra), and Ultrabithorax (Ubx). Although WTAP has been found in functional human spliceosomes, exactly how it contributes to the splicing process remains unknown. Here we attempt to identify factors that interact genetically and physically with fl(2)d. We begin by analyzing the Sxl-Fl(2)d protein-protein interaction in detail and present evidence suggesting that the female-specific fl(2)d(1) allele is antimorphic with respect to the process of sex determination. Next we show that fl(2)d interacts genetically with early acting general splicing regulators and that Fl(2)d is present in immunoprecipitable complexes with Snf, U2AF50, U2AF38, and U1-70K. By contrast, we could not detect Fl(2)d complexes containing the U5 snRNP protein U5-40K or with a protein that associates with the activated B spliceosomal complex SKIP. Significantly, the genetic and molecular interactions observed for Sxl are quite similar to those detected for fl(2)d. Taken together, our findings suggest that Sxl and fl(2)d function to alter splice-site selection at an early step in spliceosome assembly.
Figures


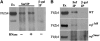

Similar articles
-
PPS, a large multidomain protein, functions with sex-lethal to regulate alternative splicing in Drosophila.PLoS Genet. 2010 Mar 5;6(3):e1000872. doi: 10.1371/journal.pgen.1000872. PLoS Genet. 2010. PMID: 20221253 Free PMC article.
-
The translation initiation factor eIF4E regulates the sex-specific expression of the master switch gene Sxl in Drosophila melanogaster.PLoS Genet. 2011 Jul;7(7):e1002185. doi: 10.1371/journal.pgen.1002185. Epub 2011 Jul 28. PLoS Genet. 2011. PMID: 21829374 Free PMC article.
-
Biochemical function of female-lethal (2)D/Wilms' tumor suppressor-1-associated proteins in alternative pre-mRNA splicing.J Biol Chem. 2003 Jan 31;278(5):3040-7. doi: 10.1074/jbc.M210737200. Epub 2002 Nov 19. J Biol Chem. 2003. PMID: 12444081
-
Vascular biology and the sex of flies: regulation of vascular smooth muscle cell proliferation by wilms' tumor 1-associating protein.Trends Cardiovasc Med. 2007 Oct;17(7):230-4. doi: 10.1016/j.tcm.2007.08.002. Trends Cardiovasc Med. 2007. PMID: 17936204 Review.
-
Masters change, slaves remain.Bioessays. 2003 Jan;25(1):1-4. doi: 10.1002/bies.10207. Bioessays. 2003. PMID: 12508274 Review.
Cited by
-
spenito is required for sex determination in Drosophila melanogaster.Proc Natl Acad Sci U S A. 2015 Sep 15;112(37):11606-11. doi: 10.1073/pnas.1515891112. Epub 2015 Aug 31. Proc Natl Acad Sci U S A. 2015. PMID: 26324914 Free PMC article.
-
Sex determination in Drosophila: The view from the top.Fly (Austin). 2010 Jan-Mar;4(1):60-70. doi: 10.4161/fly.4.1.11277. Epub 2010 Jan 21. Fly (Austin). 2010. PMID: 20160499 Free PMC article. Review.
-
N6-methyladenosine modifications: interactions with novel RNA-binding proteins and roles in signal transduction.RNA Biol. 2019 Aug;16(8):991-1000. doi: 10.1080/15476286.2019.1620060. Epub 2019 May 26. RNA Biol. 2019. PMID: 31107151 Free PMC article. Review.
-
PPS, a large multidomain protein, functions with sex-lethal to regulate alternative splicing in Drosophila.PLoS Genet. 2010 Mar 5;6(3):e1000872. doi: 10.1371/journal.pgen.1000872. PLoS Genet. 2010. PMID: 20221253 Free PMC article.
-
Nuclear degradation of Wilms tumor 1-associating protein and survivin splice variant switching underlie IGF-1-mediated survival.J Biol Chem. 2009 Sep 11;284(37):24684-95. doi: 10.1074/jbc.M109.034629. Epub 2009 Jul 15. J Biol Chem. 2009. PMID: 19605357 Free PMC article.
References
-
- Achsel, T., K. Ahrens, H. Brahams, S. Teigelkamp and R. Luhrmann, 1998. The human U5–220kD protein (hPrp8) forms a stable RNA-free complex with several U5-specific proteins, including an RNA unwindase, a homologue of ribosomal elongation factor EF-2, and a novel WD-40 protein. Mol. Cell. Biol. 18 6756–6766. - PMC - PubMed
-
- Cline, T., and B. Meyer, 1996. Viva la difference: males vs females in flies vs worms. Annu. Rev. Genet. 30 637–702. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

