Stressed out: the skeletal muscle ryanodine receptor as a target of stress
- PMID: 18246195
- PMCID: PMC2214709
- DOI: 10.1172/JCI34006
Stressed out: the skeletal muscle ryanodine receptor as a target of stress
Abstract
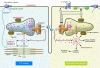
Over the past century, understanding the mechanisms underlying muscle fatigue and weakness has been the focus of much investigation. However, the dominant theory in the field, that lactic acidosis causes muscle fatigue, is unlikely to tell the whole story. Recently, dysregulation of sarcoplasmic reticulum (SR) Ca(2+) release has been associated with impaired muscle function induced by a wide range of stressors, from dystrophy to heart failure to muscle fatigue. Here, we address current understandings of the altered regulation of SR Ca(2+) release during chronic stress, focusing on the role of the SR Ca(2+) release channel known as the type 1 ryanodine receptor.
Figures

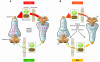
References
-
- Tanabe T., Beam K.G., Adams B.A., Niidome T., Numa S. Regions of the skeletal muscle dihydropyridine receptor critical for excitation-contraction coupling. Nature. 1990;346:567–569. - PubMed
-
- Fill M., Copello J.A. Ryanodine receptor calcium release channels. Physiol. Rev. 2002;82:893–922. - PubMed
-
- Gonzalez-Serratos H., Valle-Aguilera R., Lathrop D.A., Garcia M.C. Slow inward calcium currents have no obvious role in muscle excitation-contraction coupling. Nature. 1982;298:292–294. - PubMed
-
- Rios E., Brum G. Involvement of dihydropyridine receptors in excitation-contraction coupling in skeletal muscle. Nature. 1987;325:717–720. - PubMed
-
- Catterall W.A. Excitation-contraction coupling in vertebrate skeletal muscle: a tale of two calcium channels. Cell. 1991;64:871–874. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

