Aquaporin 1 - a novel player in spinal cord injury
- PMID: 18248364
- PMCID: PMC2634749
- DOI: 10.1111/j.1471-4159.2007.05177.x
Aquaporin 1 - a novel player in spinal cord injury
Abstract
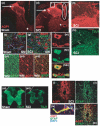
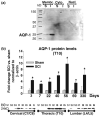
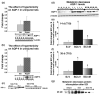
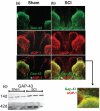
The role of water channel aquaporin 1 (AQP-1) in uninjured or injured spinal cords is unknown. AQP-1 is weakly expressed in neurons and gray matter astrocytes, and more so in white matter astrocytes in uninjured spinal cords, a novel finding. As reported before, AQP-1 is also present in ependymal cells, but most abundantly in small diameter sensory fibers of the dorsal horn. Rat contusion spinal cord injury (SCI) induced persistent and significant four- to eightfold increases in AQP-1 levels at the site of injury (T10) persisting up to 11 months post-contusion, a novel finding. Delayed AQP-1 increases were also found in cervical and lumbar segments, suggesting the spreading of AQP-1 changes over time after SCI. Given that the antioxidant melatonin significantly decreased SCI-induced AQP-1 increases and that hypoxia inducible factor-1alpha was increased in acutely and chronically injured spinal cords, we propose that chronic hypoxia contributes to persistent AQP-1 increases after SCI. Interestingly; AQP-1 levels were not affected by long-lasting hypertonicity that significantly increased astrocytic AQP-4, suggesting that the primary role of AQP-1 is not regulating isotonicity in spinal cords. Based on our results we propose possible novel roles for AQP-1 in the injured spinal cords: (i) in neuronal and astrocytic swelling, as AQP-1 was increased in all surviving neurons and reactive astrocytes after SCI and (ii) in the development of the neuropathic pain after SCI. We have shown that decreased AQP-1 in melatonin-treated SCI rats correlated with decreased AQP-1 immunolabeling in the dorsal horns sensory afferents, and with significantly decreased mechanical allodynia, suggesting a possible link between AQP-1 and chronic neuropathic pain after SCI.
Figures

Similar articles
-
Acute and chronic changes in aquaporin 4 expression after spinal cord injury.Neuroscience. 2006 Dec;143(3):779-92. doi: 10.1016/j.neuroscience.2006.08.079. Epub 2006 Oct 30. Neuroscience. 2006. PMID: 17074445 Free PMC article.
-
Upregulation of calcium channel alpha-2-delta-1 subunit in dorsal horn contributes to spinal cord injury-induced tactile allodynia.Spine J. 2018 Jun;18(6):1062-1069. doi: 10.1016/j.spinee.2018.01.010. Epub 2018 Jan 31. Spine J. 2018. PMID: 29355786
-
Aquaporins in spinal cord injury: the janus face of aquaporin 4.Neuroscience. 2010 Jul 28;168(4):1019-35. doi: 10.1016/j.neuroscience.2010.01.037. Epub 2010 Jan 28. Neuroscience. 2010. PMID: 20109536 Free PMC article. Review.
-
Acute exercise prevents the development of neuropathic pain and the sprouting of non-peptidergic (GDNF- and artemin-responsive) c-fibers after spinal cord injury.Exp Neurol. 2014 May;255:38-48. doi: 10.1016/j.expneurol.2014.02.013. Epub 2014 Feb 19. Exp Neurol. 2014. PMID: 24560714 Free PMC article.
-
The role of aquaporin 4 (AQP4) in spinal cord injury.Biomed Pharmacother. 2022 Jan;145:112384. doi: 10.1016/j.biopha.2021.112384. Epub 2021 Dec 6. Biomed Pharmacother. 2022. PMID: 34915672 Review.
Cited by
-
Vascular endothelial growth factor and spinal cord injury pain.J Neurotrauma. 2010 Oct;27(10):1793-803. doi: 10.1089/neu.2010.1351. J Neurotrauma. 2010. PMID: 20698758 Free PMC article.
-
Amiloride improves locomotor recovery after spinal cord injury.J Neurotrauma. 2011 Jul;28(7):1319-26. doi: 10.1089/neu.2011.1921. J Neurotrauma. 2011. PMID: 21534729 Free PMC article.
-
Investigation of Neuroprotective Effects of Erythropoietin on Chronic Neuropathic Pain in a Chronic Constriction Injury Rat Model.J Pain Res. 2020 Nov 30;13:3147-3155. doi: 10.2147/JPR.S285870. eCollection 2020. J Pain Res. 2020. PMID: 33311994 Free PMC article.
-
A systematic study of the distribution and expression of aquaporin water channels in normal adult human brain.J Neurol. 2025 Aug 13;272(9):574. doi: 10.1007/s00415-025-13304-9. J Neurol. 2025. PMID: 40801970
-
Curcumin attenuates brain edema in mice with intracerebral hemorrhage through inhibition of AQP4 and AQP9 expression.Acta Pharmacol Sin. 2015 Aug;36(8):939-48. doi: 10.1038/aps.2015.47. Epub 2015 Jun 29. Acta Pharmacol Sin. 2015. PMID: 26119880 Free PMC article.
References
-
- Allegra M, Reiter RJ, Tan DX, Gentile C, Tesoriere L, Livrea MA. The chemistry of melatonin's interaction with reactive species. J. Pineal Res. 2003;34:1–10. - PubMed
-
- Amiry-Moghaddam M, Lindland H, Zelenin S, Roberg BA, Gundersen BB, Petersen P, Rinvik E, Torgner IA, Ottersen OP. Brain mitochondria contain aquaporin water channels: evidence for the expression of a short AQP9 isoform in the inner mitochondrial membrane. FASEB J. 2005;19:1459–1467. - PubMed
-
- Basaldella L, Orvieto E, Dei Tos AP, Della Barbera M, Valente M, Longatti P. Causes of arachnoid cyst development and expansion. Neurosurg. Focus. 2007;22:00. - PubMed
-
- Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J. Neurotrauma. 1995;12:1–21. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

