HIF-1 alpha is an essential effector for purine nucleoside-mediated neuroprotection against hypoxia in PC12 cells and primary cerebellar granule neurons
- PMID: 18248612
- PMCID: PMC2992945
- DOI: 10.1111/j.1471-4159.2008.05275.x
HIF-1 alpha is an essential effector for purine nucleoside-mediated neuroprotection against hypoxia in PC12 cells and primary cerebellar granule neurons
Abstract
Hypoxia-inducible factor-1 alpha (HIF-1alpha) and purine nucleosides adenosine and inosine are critical mediators of physiological responses to acute and chronic hypoxia. The specific aim of this paper was to evaluate the potential role of HIF-1alpha in purine-mediated neuroprotection. We show that adenosine and inosine efficiently rescued clonal rat pheochromocytoma (PC12) cells (up to 43.6%) as well as primary cerebellar granule neurons (up to 25.1%) from hypoxic insult, and furthermore, that HIF-1alpha is critical for purine-mediated neuroprotection. Next, we studied hypoxia or purine nucleoside increased nuclear accumulation of HIF-1alpha in PC12 cells. As a possible result of increased protein stabilization or synthesis an up to 2.5-fold induction of HIF-1alpha accumulation was detected. In cerebellar granule neurons, purine nucleosides induced an up to 3.1-fold HIF-1alpha accumulation in cell lysates. Concomitant with these results, small interfering RNA-mediated reduction of HIF-1alpha completely abolished adenosine- and inosine-mediated protection in PC12 cells and severely hampered purine nucleoside-mediated protection in primary neurons (up to 94.2%). Data presented in this paper thus clearly demonstrate that HIF-1alpha is a key regulator of purine nucleoside-mediated rescue of hypoxic neuronal cells.
Figures
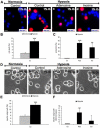





References
-
- Arslan G, Kull B, Fredholm BB. Signaling via A2A adenosine receptor in four PC12 cell clones. Naunyn Schmiedebergs Arch Pharmacol. 1999;359:28–32. - PubMed
-
- Ballarin M, Fredholm BB, Ambrosio S, Mahy N. Extracellular levels of adenosine and its metabolites in the striatum of awake rats: inhibition of uptake and metabolism. Acta Physiol Scand. 1991;142:97–103. - PubMed
-
- Benowitz LI, Jing Y, Tabibiazar R, Jo SA, Petrausch B, Stuermer CA, Rosenberg PA, Irwin N. Axon outgrowth is regulated by an intracellular purine-sensitive mechanism in retinal ganglion cells. J Biol Chem. 1998;273:29626–29634. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

