N,N-dimethyl-thioamphetamine and methyl-thioamphetamine, two non-neurotoxic substrates of 5-HT transporters, have scant in vitro efficacy for the induction of transporter-mediated 5-HT release and currents
- PMID: 18248615
- PMCID: PMC4502523
- DOI: 10.1111/j.1471-4159.2008.05272.x
N,N-dimethyl-thioamphetamine and methyl-thioamphetamine, two non-neurotoxic substrates of 5-HT transporters, have scant in vitro efficacy for the induction of transporter-mediated 5-HT release and currents
Abstract
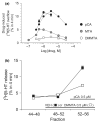
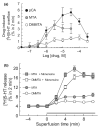
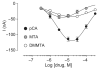
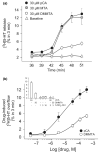
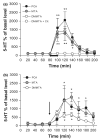
We studied two non-neurotoxic amphetamine derivatives (methyl-thioamphetamine, MTA and N,N-dimethylMTA, DMMTA) interacting with serotonin (5-HT) transporters (SERTs) with affinities comparable to that of p-Cl-amphetamine (pCA). The rank order for their maximal effects in inducing both [(3)H]5-HT release from rat brain synaptosomes or hSERT-expressing HEK-293 cells, and currents in hSERT-expressing oocytes, was pCA >> MTA > or = DMMTA. A correlation between drug-induced release and currents is also strengthened by the similar bell shape of the dose-response curves. Release experiments indicated that MTA and DMMTA are SERT substrates although MTA is taken up by HEK-293 cells with a V(max) 40% lower than pCA. The weak effects of MTA and DMMTA in vitro might therefore be due to their properties as 'partial substrates' on the mechanisms, other than translocation, responsible for currents and/or release. After either local or systemic in vivo administration, MTA and DMMTA release 5-HT in a manner comparable to pCA. These findings confirm that the neurotoxic properties of some amphetamine derivatives are independent of their 5-HT-releasing activity in vivo. It is worth noting that only those amphetamine derivatives with high efficiency in inducing 5-HT release and currents in vitro have neurotoxic properties.
Figures


References
-
- Baumann MH, Ayestas MA, Dersch CM, Rothman RB. 1-(m-chlorophenyl)piperazine (mCPP) dissociates in vivo serotonin release from long-term serotonin depletion in rat brain. Neuropsychopharmacology. 2001;24:492–501. - PubMed
-
- Dinopoulos A, Dori I, Parnavelas JG. Serotonergic innervation of the mature and developing lateral septum of the rat: a light and electron microscopic immunocytochemical analysis. Neuroscience. 1993;55:209–222. - PubMed
-
- Eriksson E, Engberg G, Bing O, Nissbrandt H. Effects of mCPP on the extracellular concentrations of serotonin and dopa-mine in rat brain. Neuropsychopharmacology. 1999;20:287–296. - PubMed
-
- Fischer JF, Cho AK. Chemical release of dopamine from striatal homogenates: evidence for an exchange diffusion model. J. Pharmacol. Exp. Ther. 1979;208:203–209. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

