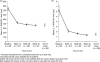Control of IGF-I levels with titrated dosing of lanreotide Autogel over 48 weeks in patients with acromegaly
- PMID: 18248639
- PMCID: PMC2610402
- DOI: 10.1111/j.1365-2265.2008.03208.x
Control of IGF-I levels with titrated dosing of lanreotide Autogel over 48 weeks in patients with acromegaly
Abstract
Background: An essential criterion for control of acromegaly is normalization of IGF-I levels. Somatostatin analogues act to suppress IGF-I and GH levels.
Objective: To assess the efficacy and safety of 48 weeks titrated dosing of lanreotide Autogel.
Design: Open-label, multicentre, phase III, 48-week trial.
Methods: Patients with active acromegaly (IGF-I levels > 1.3 times upper limit of age-adjusted normal range) were recruited. Twelve injections of lanreotide Autogel were given at 28-day intervals: during the 16-week fixed-dose phase, patients received 90 mg; in the 32-week dose-titration phase, patients received 60, 90 or 120 mg according to GH and IGF-I levels. Intention-to-treat analysis was performed to determine the proportion of patients with normalized age-adjusted IGF-I levels at study end. Secondary evaluations included GH levels, clinical acromegaly signs and safety.
Results: Fifty-seven of 63 patients completed the study. Lanreotide Autogel resulted in normalized age-adjusted IGF-I levels in 27 patients (43%, 95% CI 31-55). Mean GH levels decreased from 6.2 to 1.5 microg/l at study end, with 53 of 62 patients (85%) having GH levels < or = 2.5 microg/l (95% CI 76.7-94.3) and 28 of 62 patients (45%) with levels < 1 microg/l (95% CI 32.8-57.6). Twenty-four (38%) had both normal IGF-I levels and GH levels < or = 2.5 microg/l. Acromegaly symptoms reduced significantly in most patients throughout the study. The most common adverse events were gastrointestinal, as expected for somatostatin analogues.
Conclusions: Using IGF-I as primary end-point, 48 weeks lanreotide Autogel treatment, titrated for optimal hormonal control, controlled IGF-I and GH levels effectively, reduced acromegaly symptoms and was well tolerated.
Figures
References
-
- Melmed S, Casanueva F, Cavagnini F, Chanson P, Frohman LA, Gaillard R, Ghigo E, Ho K, Jaquet P, Kleinberg D, Lamberts S, Laws E, Lombardi G, Sheppard MC, Thorner M, Vance ML, Wass JA, Giustina A. Consensus statement: medical management of acromegaly. European Journal of Endocrinology. 2005;153:737–740. - PubMed
-
- Colao A, Ferone D, Marzullo P, Lombardi G. Systemic complications of acromegaly: epidemiology, pathogenesis, and management. Endocrine Reviews. 2004;25:102–152. - PubMed
-
- Holdaway IM, Rajasoorya C, Gamble GD. Factors influencing mortality in acromegaly. Journal of Clinical Endocrinology and Metabolism. 2004;89:667–674. - PubMed
-
- Melmed S. Medical progress: acromegaly. New England Journal of Medicine. 2006;355:2558–2573. - PubMed
-
- Caron P, Cogne M, Raingeard I, Bex-Bachellerie V, Kuhn JM. Effectiveness and tolerability of 3-year lanreotide Autogel treatment in patients with acromegaly. Clinical Endocrinology. 2006;64:209–214. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical