Age-related intrinsic changes in human bone-marrow-derived mesenchymal stem cells and their differentiation to osteoblasts
- PMID: 18248663
- PMCID: PMC2398731
- DOI: 10.1111/j.1474-9726.2008.00377.x
Age-related intrinsic changes in human bone-marrow-derived mesenchymal stem cells and their differentiation to osteoblasts
Abstract
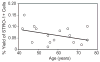
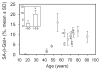
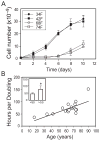
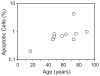
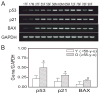
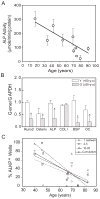
In vivo and in vitro studies indicate that a subpopulation of human marrow-derived stromal cells (MSCs, also known as mesenchymal stem cells) has potential to differentiate into multiple cell types, including osteoblasts. In this study, we tested the hypothesis that there are intrinsic effects of age in human MSCs (17-90 years). We tested the effect of age on senescence-associated beta-galactosidase, proliferation, apoptosis, p53 pathway genes, and osteoblast differentiation in confluent monolayers by alkaline phosphatase activity and osteoblast gene expression analysis. There were fourfold more human bone MSCs (hMSCs) positive for senescence-associated beta-galactosidase in samples from older than younger subjects (P < 0.001; n = 17). Doubling time of hMSCs was 1.7-fold longer in cells from the older than the younger subjects, and was positively correlated with age (P = 0.002; n = 19). Novel age-related changes were identified. With age, more cells were apoptotic (P = 0.016; n = 10). Further, there were age-related increases in expression of p53 and its pathway genes, p21 and BAX. Consistent with other experiments, there was a significant age-related decrease in generation of osteoblasts both in the STRO-1+ cells (P = 0.047; n = 8) and in adherent MSCs (P < 0.001; n = 10). In sum, there is an age-dependent decrease in proliferation and osteoblast differentiation, and an increase in senescence-associated beta-galactosidase-positive cells and apoptosis in hMSCs. Up-regulation of the p53 pathway with age may have a critical role in mediating the reduction in both proliferation and osteoblastogenesis of hMSCs. These findings support the view that there are intrinsic alterations in human MSCs with aging that may contribute to the process of skeletal aging in humans.
Figures
Similar articles
-
Effects of age on parathyroid hormone signaling in human marrow stromal cells.Aging Cell. 2011 Oct;10(5):780-8. doi: 10.1111/j.1474-9726.2011.00717.x. Epub 2011 May 25. Aging Cell. 2011. PMID: 21518242 Free PMC article.
-
Replicative senescence of human bone marrow and umbilical cord derived mesenchymal stem cells and their differentiation to adipocytes and osteoblasts.Mol Biol Rep. 2011 Nov;38(8):5161-8. doi: 10.1007/s11033-010-0665-2. Epub 2010 Dec 29. Mol Biol Rep. 2011. PMID: 21188535
-
Wnt/β-catenin signaling mediates the senescence of bone marrow-mesenchymal stem cells from systemic lupus erythematosus patients through the p53/p21 pathway.Mol Cell Biochem. 2014 Feb;387(1-2):27-37. doi: 10.1007/s11010-013-1866-5. Epub 2013 Oct 16. Mol Cell Biochem. 2014. PMID: 24130040
-
Vitamin D metabolism in human bone marrow stromal (mesenchymal stem) cells.Metabolism. 2013 Jun;62(6):768-77. doi: 10.1016/j.metabol.2013.01.003. Epub 2013 Jan 30. Metabolism. 2013. PMID: 23375059 Free PMC article. Review.
-
Osteoblastic cells: differentiation and trans-differentiation.Arch Biochem Biophys. 2008 May 15;473(2):183-7. doi: 10.1016/j.abb.2008.03.028. Epub 2008 Mar 29. Arch Biochem Biophys. 2008. PMID: 18406334 Review.
Cited by
-
Geriatric fragility fractures are associated with a human skeletal stem cell defect.Aging Cell. 2020 Jul;19(7):e13164. doi: 10.1111/acel.13164. Epub 2020 Jun 14. Aging Cell. 2020. PMID: 32537886 Free PMC article.
-
Global transcriptional profiling using RNA sequencing and DNA methylation patterns in highly enriched mesenchymal cells from young versus elderly women.Bone. 2015 Jul;76:49-57. doi: 10.1016/j.bone.2015.03.017. Epub 2015 Mar 27. Bone. 2015. PMID: 25827254 Free PMC article.
-
Mesenchymal Stromal Cells Implantation in Combination with Platelet Lysate Product Is Safe for Reconstruction of Human Long Bone Nonunion.Cell J. 2016 Fall;18(3):302-309. doi: 10.22074/cellj.2016.4557. Epub 2016 Aug 24. Cell J. 2016. PMID: 27602311 Free PMC article.
-
Mesenchymal stem cell aging: Mechanisms and influences on skeletal and non-skeletal tissues.Exp Biol Med (Maywood). 2015 Aug;240(8):1099-106. doi: 10.1177/1535370215591828. Epub 2015 Jun 18. Exp Biol Med (Maywood). 2015. PMID: 26088863 Free PMC article. Review.
-
Mesenchymal stem cells as a potent cell source for bone regeneration.Stem Cells Int. 2012;2012:980353. doi: 10.1155/2012/980353. Epub 2012 Feb 16. Stem Cells Int. 2012. PMID: 22448175 Free PMC article.
References
-
- Artandi SE, Attardi LD. Pathways connecting telomeres and p53 in senescence, apoptosis, and cancer. Biochem Biophys Res Commun. 2005;331:881–890. - PubMed
-
- Bobis S, Jarocha D, Majka M. Mesenchymal stem cells: characteristics and clinical applications. Folia Histochem Cytobiol. 2006;44:215–230. - PubMed
-
- Bird J, Ostler EL, Faragher RG. Can we say that senescent cells cause ageing? Exp Gerontol. 2003;38:1319–1326. - PubMed
-
- Campisi J. The role of cellular senescence in skin aging. J Investig Dermatol Symp Proc. 1998;3:1–5. - PubMed
-
- Campisi J. Senescent cells, tumor suppression, and organismal aging: Good citizens, bad neighbors. Cell. 2005;120:513–522. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

