Age-related intrinsic changes in human bone-marrow-derived mesenchymal stem cells and their differentiation to osteoblasts
- PMID: 18248663
- PMCID: PMC2398731
- DOI: 10.1111/j.1474-9726.2008.00377.x
Age-related intrinsic changes in human bone-marrow-derived mesenchymal stem cells and their differentiation to osteoblasts
Abstract
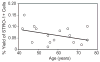
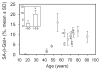
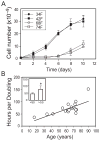
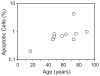
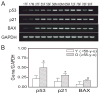
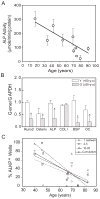
In vivo and in vitro studies indicate that a subpopulation of human marrow-derived stromal cells (MSCs, also known as mesenchymal stem cells) has potential to differentiate into multiple cell types, including osteoblasts. In this study, we tested the hypothesis that there are intrinsic effects of age in human MSCs (17-90 years). We tested the effect of age on senescence-associated beta-galactosidase, proliferation, apoptosis, p53 pathway genes, and osteoblast differentiation in confluent monolayers by alkaline phosphatase activity and osteoblast gene expression analysis. There were fourfold more human bone MSCs (hMSCs) positive for senescence-associated beta-galactosidase in samples from older than younger subjects (P < 0.001; n = 17). Doubling time of hMSCs was 1.7-fold longer in cells from the older than the younger subjects, and was positively correlated with age (P = 0.002; n = 19). Novel age-related changes were identified. With age, more cells were apoptotic (P = 0.016; n = 10). Further, there were age-related increases in expression of p53 and its pathway genes, p21 and BAX. Consistent with other experiments, there was a significant age-related decrease in generation of osteoblasts both in the STRO-1+ cells (P = 0.047; n = 8) and in adherent MSCs (P < 0.001; n = 10). In sum, there is an age-dependent decrease in proliferation and osteoblast differentiation, and an increase in senescence-associated beta-galactosidase-positive cells and apoptosis in hMSCs. Up-regulation of the p53 pathway with age may have a critical role in mediating the reduction in both proliferation and osteoblastogenesis of hMSCs. These findings support the view that there are intrinsic alterations in human MSCs with aging that may contribute to the process of skeletal aging in humans.
Figures
References
-
- Artandi SE, Attardi LD. Pathways connecting telomeres and p53 in senescence, apoptosis, and cancer. Biochem Biophys Res Commun. 2005;331:881–890. - PubMed
-
- Bobis S, Jarocha D, Majka M. Mesenchymal stem cells: characteristics and clinical applications. Folia Histochem Cytobiol. 2006;44:215–230. - PubMed
-
- Bird J, Ostler EL, Faragher RG. Can we say that senescent cells cause ageing? Exp Gerontol. 2003;38:1319–1326. - PubMed
-
- Campisi J. The role of cellular senescence in skin aging. J Investig Dermatol Symp Proc. 1998;3:1–5. - PubMed
-
- Campisi J. Senescent cells, tumor suppression, and organismal aging: Good citizens, bad neighbors. Cell. 2005;120:513–522. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

