Role of chimaerins, a group of Rac-specific GTPase activating proteins, in T-cell receptor signaling
- PMID: 18249095
- PMCID: PMC2277368
- DOI: 10.1016/j.cellsig.2007.12.015
Role of chimaerins, a group of Rac-specific GTPase activating proteins, in T-cell receptor signaling
Abstract
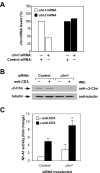
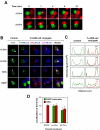
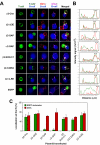
Chimaerins are GTPase-activating proteins that inactivate the GTP-hydrolase Rac1 in a diacylglycerol-dependent manner. To date, the study of chimaerins has been done mostly in neuronal cells. Here, we show that alpha2- and beta2-chimaerin are expressed at different levels in T-cells and that they participate in T-cell receptor signaling. In agreement with this, we have observed that alpha2- and beta2-chimaerins translocate to the T-cell/B-cell immune synapse and, using both gain- and loss-of-function approaches, demonstrated that their catalytic activity is important for the inhibition of the T-cell receptor- and Vav1-dependent stimulation of the transcriptional factor NF-AT. Mutagenesis-based approaches have revealed the molecular determinants that contribute to the biological program of chimaerins during T-cell responses. Unexpectedly, we have found that the translocation of chimaerins to the T-cell/B-cell immune synapse does not rely on the canonical binding of diacylglycerol to the C1 region of these GTPase-activating proteins. Taken together, these results identify chimaerins as candidates for the downmodulation of Rac1 in T-lymphocytes and, in addition, uncover a novel regulatory mechanism that mediates their activation in T-cells.
Figures




References
-
- Kane LP, Lin J, Weiss A. Curr Opin Immunol. 2000;12(3):242–249. - PubMed
-
- Clements JL, Boerth NJ, Lee JR, Koretzky GA. Annu Rev Immunol. 1999;17:89–108. - PubMed
-
- Acuto O, Cantrell D. Annu Rev Immunol. 2000;18:165–184. - PubMed
-
- Vicente-Manzanares M, Sanchez-Madrid F. Nat Rev Immunol. 2004;4(2):110–122. - PubMed
-
- Penninger JM, Crabtree GR. Cell. 1999;96(1):9–12. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

