Normal development is an integral part of tumorigenesis in T cell-specific PTEN-deficient mice
- PMID: 18250301
- PMCID: PMC2538875
- DOI: 10.1073/pnas.0712059105
Normal development is an integral part of tumorigenesis in T cell-specific PTEN-deficient mice
Abstract
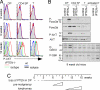
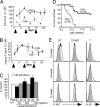
PTEN is a tumor suppressor gene but whether cancer can develop in all PTEN-deficient cells is not known. In T cell-specific PTEN-deficient (tPTEN-/-) mice, which suffer from mature T cell lymphomas, we found that premalignancy, as defined by elevated AKT and senescence pathways, starts in immature T cell precursors and surprisingly not in mature T cells. Premalignancy only starts in 6-week-old mice and becomes much stronger in 9-week-old mice although PTEN is lost since birth. tPTEN-/- immature T cells do not become tumors, and senescence has no role in this model because these cells exist in a novel cell cycle state, expressing proliferating proteins but not proliferating to any significant degree. Instead, the levels of p27(kip1), which is lower in tPTEN-/- immature T cells and almost nonexistent in tPTEN-/- mature T cells, correlate with the proliferation capability of these cells. Interestingly, transient reduction of these cancer precursor cells in adult tPTEN-/- mice within a crucial time window significantly delayed lymphomas and mouse lethality. Thus, loss of PTEN alone is not sufficient for cells to become cancerous, therefore other developmental events are necessary for tumor formation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

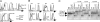

References
-
- Yilmaz OH, et al. Pten dependence distinguishes hematopoietic stem cells from leukemia-initiating cells. Nature. 2006;441:475–482. - PubMed
-
- Al-Hajj M, et al. Therapeutic implications of cancer stem cells. Curr Opin Genet Dev. 2004;14:43–47. - PubMed
-
- Kelly PN, et al. Tumor growth need not be driven by rare cancer stem cells. Science. 2007;317:337. - PubMed
-
- Cully M, You H, Levine AJ, Mak TW. Beyond PTEN mutations: The PI3K pathway as an integrator of multiple inputs during tumorigenesis. Nat Rev Cancer. 2006;6:184–192. - PubMed
-
- Samuels Y, Ericson K. Oncogenic PI3K and its role in cancer. Curr Opin Oncol. 2006;18:77–82. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

