Bcl-xL induces Drp1-dependent synapse formation in cultured hippocampal neurons
- PMID: 18250306
- PMCID: PMC2542873
- DOI: 10.1073/pnas.0711647105
Bcl-xL induces Drp1-dependent synapse formation in cultured hippocampal neurons
Abstract
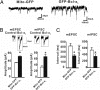
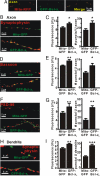
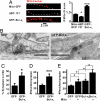
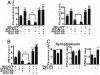
Maturation of neuronal synapses is thought to involve mitochondria. Bcl-xL protein inhibits mitochondria-mediated apoptosis but may have other functions in healthy adult neurons in which Bcl-xL is abundant. Here, we report that overexpression of Bcl-xL postsynaptically increases frequency and amplitude of spontaneous miniature synaptic currents in rat hippocampal neurons in culture. Bcl-xL, overexpressed either pre or postsynaptically, increases synapse number, the number and size of synaptic vesicle clusters, and mitochondrial localization to vesicle clusters and synapses, likely accounting for the changes in miniature synaptic currents. Conversely, knockdown of Bcl-xL or inhibiting it with ABT-737 decreases these morphological parameters. The mitochondrial fission protein, dynamin-related protein 1 (Drp1), is a GTPase known to localize to synapses and affect synaptic function and structure. The effects of Bcl-xL appear mediated through Drp1 because overexpression of Drp1 increases synaptic markers, and overexpression of the dominant-negative dnDrp1-K38A decreases them. Furthermore, Bcl-xL coimmunoprecipitates with Drp1 in tissue lysates, and in a recombinant system, Bcl-xL protein stimulates GTPase activity of Drp1. These findings suggest that Bcl-xL positively regulates Drp1 to alter mitochondrial function in a manner that stimulates synapse formation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Sanes JR, Lichtman JW. Development of the vertebrate neuromuscular junction. Annu Rev Neurosci. 1999;22:389–442. - PubMed
-
- Young SH, Poo MM. Spontaneous release of transmitter from growth cones of embryonic neurones. Nature. 1983;305:634–637. - PubMed
-
- Zhang W, Benson DL. Development and molecular organization of dendritic spines and their synapses. Hippocampus. 2000;10:512–526. - PubMed
-
- Ziv NE, Garner CC. Cellular and molecular mechanisms of presynaptic assembly. Nat Rev Neurosci. 2004;5:385–399. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

