Signaling of cell fate determination by the TPD1 small protein and EMS1 receptor kinase
- PMID: 18250314
- PMCID: PMC2538901
- DOI: 10.1073/pnas.0708795105
Signaling of cell fate determination by the TPD1 small protein and EMS1 receptor kinase
Abstract
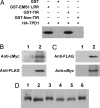
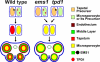
Sexual reproduction requires the specification of cells with distinct fates in plants and animals. The EMS1 (also known as EXS) leucine-rich repeat receptor-like kinase (LRR-RLK) and TPD1 small protein play key roles in regulating somatic and reproductive cell fate determination in Arabidopsis anthers. Here, we show that ectopic expression of TPD1 causes abnormal differentiation of somatic and reproductive cells in anthers. In addition, ectopic TPD1 activity requires functional EMS1. Yeast two-hybrid, pull-down, and coimmunoprecipitation analyses further demonstrate that TPD1 interacts with EMS1 in vitro and in vivo. Moreover, TPD1 induces EMS1 phosphorylation in planta. Thus, our results suggest that TPD1 serves as a ligand for the EMS1 receptor kinase to signal cell fate determination during plant sexual reproduction.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Control of Anther Cell Differentiation by the Small Protein Ligand TPD1 and Its Receptor EMS1 in Arabidopsis.PLoS Genet. 2016 Aug 18;12(8):e1006147. doi: 10.1371/journal.pgen.1006147. eCollection 2016 Aug. PLoS Genet. 2016. PMID: 27537183 Free PMC article.
-
Overexpression of TAPETUM DETERMINANT1 alters the cell fates in the Arabidopsis carpel and tapetum via genetic interaction with excess microsporocytes1/extra sporogenous cells.Plant Physiol. 2005 Sep;139(1):186-91. doi: 10.1104/pp.105.063529. Epub 2005 Jul 29. Plant Physiol. 2005. PMID: 16055681 Free PMC article.
-
Ectopic expression of TAPETUM DETERMINANT1 affects ovule development in Arabidopsis.J Exp Bot. 2016 Mar;67(5):1311-26. doi: 10.1093/jxb/erv523. Epub 2015 Dec 17. J Exp Bot. 2016. PMID: 26685185
-
Control of anther cell differentiation: a teamwork of receptor-like kinases.Sex Plant Reprod. 2009 Dec;22(4):221-8. doi: 10.1007/s00497-009-0106-3. Epub 2009 Aug 6. Sex Plant Reprod. 2009. PMID: 20033443 Review.
-
Reproductive biology: receptor-like kinases orchestrate love songs in plants.Curr Biol. 2009 Aug 11;19(15):R647-9. doi: 10.1016/j.cub.2009.06.018. Curr Biol. 2009. PMID: 19674546 Review.
Cited by
-
EMS1 and BRI1 control separate biological processes via extracellular domain diversity and intracellular domain conservation.Nat Commun. 2019 Sep 13;10(1):4165. doi: 10.1038/s41467-019-12112-w. Nat Commun. 2019. PMID: 31519884 Free PMC article.
-
Receptor-like kinases shape the plant.Nat Cell Biol. 2009 Oct;11(10):1166-73. doi: 10.1038/ncb1009-1166. Nat Cell Biol. 2009. PMID: 19794500 Review.
-
Small signaling peptides in Arabidopsis development: how cells communicate over a short distance.Plant Cell. 2012 Aug;24(8):3198-217. doi: 10.1105/tpc.112.099010. Epub 2012 Aug 28. Plant Cell. 2012. PMID: 22932676 Free PMC article. Review.
-
Co-Immunoprecipitation of Membrane-Bound Receptors.Arabidopsis Book. 2015 Jun 3;13:e0180. doi: 10.1199/tab.0180. eCollection 2015. Arabidopsis Book. 2015. PMID: 26097438 Free PMC article.
-
AMS-dependent and independent regulation of anther transcriptome and comparison with those affected by other Arabidopsis anther genes.BMC Plant Biol. 2012 Feb 15;12:23. doi: 10.1186/1471-2229-12-23. BMC Plant Biol. 2012. PMID: 22336428 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

