Resetting of FLOWERING LOCUS C expression after epigenetic repression by vernalization
- PMID: 18250331
- PMCID: PMC2542874
- DOI: 10.1073/pnas.0711453105
Resetting of FLOWERING LOCUS C expression after epigenetic repression by vernalization
Abstract
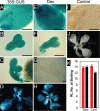
The epigenetic repression of FLOWERING LOCUS C (FLC) in winter-annual ecotypes of Arabidopsis by prolonged cold ensures that plants flower in spring and not during winter. Resetting of the FLC expression level in progeny is an important step in the life cycle of the plant. We show that both the paternally derived and the maternally derived FLC:GUS genes are reset to activity but that the timing of their first expression differs. The paternal FLC:GUS gene in vernalized plants is expressed in the male reproductive organs, the anthers, in both somatic tissue and in the sporogenous pollen mother cells, but there is no expression in mature pollen. In the progeny generation, the paternally derived FLC:GUS gene is expressed in the single-celled zygote (fertilized egg cell) and through embryo development, but not in the fertilized central cell, which generates the endosperm of the progeny seed. FLC:GUS is not expressed during female gametogenesis, with the maternally derived FLC:GUS being first expressed in the early multicellular embryo. We show that FLC activity during late embryo development is a prerequisite for the repressive action of FLC on flowering.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Helliwell CA, Wood CC, Robertson M, Peacock WJ, Dennis ES. The Arabidopsis FLC protein interacts directly in vivo with SOC1 and FT chromatin and is part of a high-molecular-weight protein complex. Plant J. 2006;46:183–192. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

