Decreases in dengue transmission may act to increase the incidence of dengue hemorrhagic fever
- PMID: 18250338
- PMCID: PMC2538904
- DOI: 10.1073/pnas.0709029105
Decreases in dengue transmission may act to increase the incidence of dengue hemorrhagic fever
Abstract
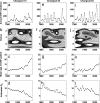
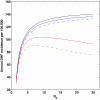
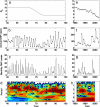
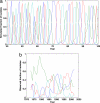
Dengue hemorrhagic fever (DHF) is a potentially fatal manifestation of an infection with the mosquito-borne dengue virus. Because of the social and economic costs of DHF, many countries in Asia and South America have initiated public health measures aimed at vector control. Despite these measures, DHF incidence rates do not appear to be declining. The effectiveness of vector control in reducing dengue transmissibility has thereby been questioned. Here, we revisit this conclusion using epidemiological data from Thailand. We first show, with age incidence data, that dengue transmission rates have fallen since 1981; surprisingly, however, these declines are not associated with decreases in DHF incidence. Instead, district-level analyses indicate a nonmonotonic relationship between the basic reproductive number R0 and DHF incidence. To understand this relationship, we formulated three mathematical models, which differ in their assumptions of transient between-serotype cross-protection. Unlike the first two models, the previously unconsidered third model with clinical cross-protection can reproduce this nonmonotonic relationship. Simulation of this model with nonstationary R0 reproduces several previously unexplained patterns of dengue dynamics, including a transition from a approximately 2-year cycle to a approximately 4-year cycle and a transient trough in DHF incidence in provinces with rapid R0 declines. These results imply that DHF incidence can be effectively controlled with a sufficiently large reduction in R0 but that moderate reductions may be counterproductive. More broadly, these results show that assuming parameter stationarity in systems with approximate stationarity in disease incidence is unjustified and may result in missed opportunities to understand the drivers of disease variability.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Gubler DJ. In: Dengue and Dengue Hemorrhagic Fever. Gubler DJ, Kuno G, editors. Wallingford, UK: CAB International; 1997. pp. 1–22.
-
- Sabin AB. Am J Trop Med Hyg. 1952;1:30–50. - PubMed
-
- Endy TP, Nisalak A, Chunsuttitwat S, Vaughn DW, Green S, Ennis FA, Rothman AL, Libraty DH. J Infect Dis. 2004;189:990–1000. - PubMed
-
- Sangkawibha N, Rojanasuphot S, Ahandrik S, Viriyapongse S, Jatanasen S, Salitul V, Phanthumachinda B, Halstead SB. Am J Epidemiol. 1984;120:653–669. - PubMed
-
- Graham RR, Juffrie M, Tan R, Hayes CG, Laksono I, Ma'roef C, Erlin, Sutaryo, Porter KR, Halstead SB. Am J Trop Med Hyg. 1999;61:412–419. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

