A CD8+/CD103high T cell subset regulates TNF-mediated chronic murine ileitis
- PMID: 18250468
- PMCID: PMC3036968
- DOI: 10.4049/jimmunol.180.4.2573
A CD8+/CD103high T cell subset regulates TNF-mediated chronic murine ileitis
Abstract
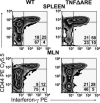
Recruitment of lymphocytes to sites of inflammation requires the sequential engagement of adhesion molecules and chemokine receptors. Of these, the lectin-like molecule CD44 has been particularly implicated in inflammatory trafficking. Using a TNF-driven model of chronic ileitis (i.e., B6.129P-Tnf(Delta)(ARE) mice) that recapitulates many features of Crohn's disease, we demonstrate dynamic changes in the expression and functional state of CD44 on CD8+ T cells. These cells coexpress CD44 and L-selectin, giving them a surface phenotype similar to that of central memory T cells. Yet functionally they exhibit the phenotype of effector T cells, because they produce IFN-gamma. Unexpectedly, depletion of the CD8+ population had no effect on the severity of ileitis. Further analyses showed a second CD8+ population that lacked CD44, but expressed CD103, produced TGF-beta, inhibited the proliferation of CD4+ in vitro, and attenuated adoptively transferred ileitis in vivo, most likely counteracting the proinflammatory role of the CD44(high) subset. Collectively, these data suggest that the presence or absence of CD44 and CD103 on the CD8+ lymphocyte surface defines functionally distinct subsets of CD8+ T cells in vivo. These inflammation-driven populations exert distinct roles during the development of chronic ileitis, and influence the balance of effector and regulatory functions in the chronically inflamed small intestine.
Figures


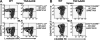



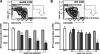

References
-
- Izcue A, Coombes JL, Powrie F. Regulatory T cells suppress systemic and mucosal immune activation to control intestinal inflammation. Immunol. Rev. 2006;212:256–271. - PubMed
-
- Mottet C, Uhlig HH, Powrie F. Cutting edge: cure of colitis by CD4+CD25+ regulatory T cells. J. Immunol. 2003;170:3939–3943. - PubMed
-
- Powrie F, Read S, Mottet C, Uhlig H, Maloy K. Control of immune pathology by regulatory T cells. Novartis Found. Symp. 2003;252:92–98. - PubMed
-
- Strober W, Fuss IJ, Blumberg RS. The immunology of mucosal models of inflammation. Annu. Rev. Immunol. 2002;20:495–549. - PubMed
-
- Fiocchi C. Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology. 1998;115:182–205. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

