A computational neuroanatomy for motor control
- PMID: 18251019
- PMCID: PMC2553854
- DOI: 10.1007/s00221-008-1280-5
A computational neuroanatomy for motor control
Abstract
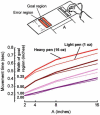
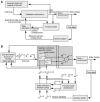
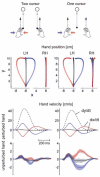
The study of patients to infer normal brain function has a long tradition in neurology and psychology. More recently, the motor system has been subject to quantitative and computational characterization. The purpose of this review is to argue that the lesion approach and theoretical motor control can mutually inform each other. Specifically, one may identify distinct motor control processes from computational models and map them onto specific deficits in patients. Here we review some of the impairments in motor control, motor learning and higher-order motor control in patients with lesions of the corticospinal tract, the cerebellum, parietal cortex, the basal ganglia, and the medial temporal lobe. We attempt to explain some of these impairments in terms of computational ideas such as state estimation, optimization, prediction, cost, and reward. We suggest that a function of the cerebellum is system identification: to build internal models that predict sensory outcome of motor commands and correct motor commands through internal feedback. A function of the parietal cortex is state estimation: to integrate the predicted proprioceptive and visual outcomes with sensory feedback to form a belief about how the commands affected the states of the body and the environment. A function of basal ganglia is related to optimal control: learning costs and rewards associated with sensory states and estimating the "cost-to-go" during execution of a motor task. Finally, functions of the primary and the premotor cortices are related to implementing the optimal control policy by transforming beliefs about proprioceptive and visual states, respectively, into motor commands.
Figures


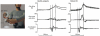


References
-
- Agostino R, Sanes JN, Hallett M. Motor skill learning in Parkinson’s disease. J Neurol Sci. 1996;139:218–226. - PubMed
-
- Bahill AT, Clark MR, Stark L. Dynamic overshoot in saccadic eye movements is caused by neurological control signed reversals. Exp Neurol. 1975;48:107–122. - PubMed
-
- Barbarulo AM, Grossi D, Merola S, Conson M, Trojano L. On the genesis of unilateral micrographia of the progressive type. Neuropsychologia. 2007;45:1685–1696. - PubMed
-
- Bastian AJ, Zackowski KM, Thach WT. Cerebellar ataxia: torque deficiency or torque mismatch between joints? J Neurophysiol. 2000;83:3019–3030. - PubMed
-
- Beer RF, Dewald JP, Rymer WZ. Deficits in the coordination of multijoint arm movements in patients with hemiparesis: evidence for disturbed control of limb dynamics. Exp Brain Res. 2000;131:305–319. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

