The use of plethysmography and oscillometry to compare long-acting bronchodilators in patients with COPD
- PMID: 18251761
- PMCID: PMC2291209
- DOI: 10.1111/j.1365-2125.2007.03013.x
The use of plethysmography and oscillometry to compare long-acting bronchodilators in patients with COPD
Abstract
What is already known about this subject: Forced expiratory volume in 1 s (FEV(1)) is the standard measurement used to measure drug effects in chronic obstructive pulmonary disease (COPD) clinical trials. Having previously shown that specific airway conductance (sGaw) measured using body plethysmography and impulse oscillometry (IOS) are more sensitive than FEV(1) for assessing short-acting bronchodilator effects in patients with COPD, we conducted the first randomized, placebo-controlled study to compare long-acting bronchodilators in COPD patients using these techniques.
What this study adds: sGaw and IOS sensitively differentiated between the effects of tiotropium and salmeterol when FEV(1) measurements were similar. sGaw and IOS measurements are better than FEV(1) for sensitively assessing bronchodilator pharmacology and differentiating between treatments in COPD clinical trials.
Aims: Assessment of bronchodilator pharmacology in chronic obstructive pulmonary disease (COPD) may be improved by using more sensitive methods than spirometry, such as impulse oscillometry (IOS) and body plethysmography. We sought to compare salmeterol (S) and tiotropium (Tio) using these methods.
Methods: In this double-blind, randomized, four-way crossover study, 32 COPD patients received single doses of Tio (18 microg), S (50 and 100 microg) or placebo. Specific airway conductance (sGaw), forced expiratory volume in 1 s (FEV(1)) and IOS were measured pre- and up to 26 h postdose. Comparisons between treatments were analysed by weighted means (WM) between 0 and 12 (WM 0-12 h) and 12-24 h (WM 12-24 h) postdose. Data are expressed as mean difference (or geometric ratio for nonparametric data) with 95% confidence intervals.
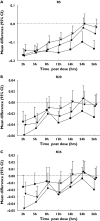
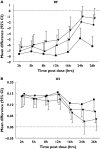
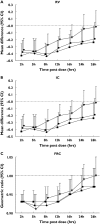
Results: Tio and S100 significantly improved FEV(1), sGaw and IOS parameters up to 26 h and S50 up to 16 h. WM analysis showed no difference between Tio and S100 in FEV(1) for 0-12 h or 12-24 h. Maximum mid-expiratory flow (-0.06; -0.11, -0.01) and R35 (0.02; 0.01, 0.03) demonstrated superiority of S100 compared with Tio for WM 0-12 h sGaw (1.12; 1.02, 1.23), R5 (-0.06; -0.09, -0.02), R15 (-0.03; -0.05, -0.01), and resonant frequency (RF) (-2.30; -3.83, -0.77) showed superiority of Tio compared with S100 for WM 12-24 h. At 26 h, sGaw, R5, R15, X5 and RF also showed superiority of Tio compared with S100.
Conclusions: sGaw and IOS parameters sensitively differentiated between the effects of Tio and S when FEV(1) measurements were similar. Clinical trials in patients with COPD should use IOS and sGaw to assess comprehensively bronchodilator pharmacology.
Figures

References
-
- Mahler DA, Donohue JF, Barbee RA, Goldman MD, Gross NJ, Wisniewski ME, Yancey SW, Zakes BA, Rickard KA, Anderson WH. Efficacy of salmeterol xinafoate in the treatment of COPD. Chest. 1999;115:957–65. - PubMed
-
- Vincken W, van Noord JA, Greefhorst AP, Bantje TA, Kesten S, Korducki L, Cornelissen PJ. Dutch/Belgian Tiotropium Study Group. Improved health outcomes in patients with COPD during 1 yr's treatment with tiotropium. Eur Respir J. 2002;19:209–16. - PubMed
-
- Newton MF, O'Donnell DE, Forkert L. Response of lung volumes to inhaled salbutamol in a large population of patients with severe hyperinflation. Chest. 2002;121:1042–50. - PubMed
-
- Celli B, ZuWallack R, Wang S, Kesten S. Improvement in resting inspiratory capacity and hyperinflation with tiotropium in COPD patients with increased static lung volumes. Chest. 2003;124:1743–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

