Mutations in BMP4 cause eye, brain, and digit developmental anomalies: overlap between the BMP4 and hedgehog signaling pathways
- PMID: 18252212
- PMCID: PMC2427285
- DOI: 10.1016/j.ajhg.2007.09.023
Mutations in BMP4 cause eye, brain, and digit developmental anomalies: overlap between the BMP4 and hedgehog signaling pathways
Abstract
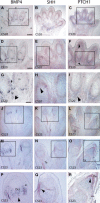
Developmental ocular malformations, including anophthalmia-microphthalmia (AM), are heterogeneous disorders with frequent sporadic or non-Mendelian inheritance. Recurrent interstitial deletions of 14q22-q23 have been associated with AM, sometimes with poly/syndactyly and hypopituitarism. We identify two further cases of AM (one with associated pituitary anomalies) with a 14q22-q23 deletion. Using a positional candidate gene approach, we analyzed the BMP4 (Bone Morphogenetic Protein-4) gene and identified a frameshift mutation (c.226del2, p.S76fs104X) that segregated with AM, retinal dystrophy, myopia, brain anomalies, and polydactyly in a family and a nonconservative missense mutation (c.278A-->G, p.E93G) in a highly conserved base in another family. MR imaging and tractography in the c.226del2 proband revealed a primary brain developmental disorder affecting thalamostriatal and callosal pathways, also present in the affected grandmother. Using in situ hybridization in human embryos, we demonstrate expression of BMP4 in optic vesicle, developing retina and lens, pituitary region, and digits strongly supporting BMP4 as a causative gene for AM, pituitary, and poly/syndactyly. Because BMP4 interacts with HH signaling genes in animals, we evaluated gene expression in human embryos and demonstrate cotemporal and cospatial expression of BMP4 and HH signaling genes. We also identified four cases, some of whom had retinal dystrophy, with "low-penetrant" mutations in both BMP4 and HH signaling genes: SHH (Sonic Hedgehog) or PTCH1 (Patched). We propose that BMP4 is a major gene for AM and/or retinal dystrophy and brain anomalies and may be a candidate gene for myopia and poly/syndactyly. Our finding of low-penetrant variants in BMP4 and HH signaling partners is suggestive of an interaction between the two pathways in humans.
Figures

 or
or  = myopia;
= myopia;  or
or  = anophthalmia, microphthalmia and retinal dystrophy;
= anophthalmia, microphthalmia and retinal dystrophy;  or
or  = polydactyly; and
= polydactyly; and  or
or  = brain anomalies. Shown in (Cviii), the right hand of case 3's grandmother with webbing.
= brain anomalies. Shown in (Cviii), the right hand of case 3's grandmother with webbing.


References
-
- Clementi M., Turolla L., Mammi I., Tenconi R. Clinical anophthalmia: An epidemiological study in northeast Italy based on 368,256 consecutive births. Teratology. 1992;46:551–553. - PubMed
-
- Ahmad M.E., Dada R., Dada T., Kucheria K. 14q (22) deletion in a familial case of anophthalmia with polydactyly. Am. J. Med. Genet. 2003;120A:117–122. - PubMed
-
- Nolen L.D., Amor D., Haywood A., St Heaps L., Willcock C., Mihelec M., Tam P., Billson F., Grigg J., Peters G. Deletion at 14q22–23 indicates a contiguous gene syndrome comprising anophthalmia, pituitary hypoplasia, and ear anomalies. Am. J. Med. Genet. A. 2006;140:1711–1718. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

