Influence of alternative thresholds for initiating HIV treatment on quality-adjusted life expectancy: a decision model
- PMID: 18252681
- PMCID: PMC3124094
- DOI: 10.7326/0003-4819-148-3-200802050-00004
Influence of alternative thresholds for initiating HIV treatment on quality-adjusted life expectancy: a decision model
Abstract
Background: The optimal threshold for initiating HIV treatment is unclear.
Objective: To compare different thresholds for initiating HIV treatment.
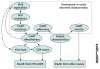
Design: A validated computer simulation was used to weigh important harms from earlier initiation of antiretroviral therapy (toxicity, side effects, and resistance accumulation) against important benefits (decreased HIV-related mortality).
Data sources: Veterans Aging Cohort Study (5742 HIV-infected patients and 11 484 matched uninfected controls) and published reports.
Target population: Individuals with newly diagnosed chronic HIV infection and varying viral loads (10,000, 30,000, 100,000, and 300,000 copies/mL) and ages (30, 40, and 50 years).
Time horizon: Unlimited.
Perspective: Societal.
Intervention: Alternative thresholds for initiating antiretroviral therapy (CD4 counts of 200, 350, and 500 cells/mm3).
Outcome measures: Life-years and quality-adjusted life-years (QALYs).
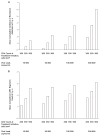
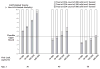
Results of base-case analysis: Although the simulation was biased against earlier treatment initiation because it used an upper-bound assumption for therapy-related toxicity, earlier treatment increased life expectancy and QALYs at age 30 years regardless of viral load (life expectancies with CD4 initiation thresholds of 500, 350, and 200 cells/mm3 were 18.2 years, 17.6 years, and 17.2 years, respectively, for a viral load of 10,000 copies/mL and 17.3 years, 15.9 years, and 14.5 years, respectively, for a viral load of 300,000 copies/mL), and increased life expectancies at age 40 years if viral loads were greater than 30 000 copies/mL (life expectancies were 12.5 years, 12.0 years, and 11.4 years, respectively, for a viral load of 300,000 copies/mL).
Results of sensitivity analysis: Findings favoring early treatment were generally robust.
Limitations: Results favoring later treatment may not be valid. The findings may not be generalizable to women.
Conclusion: This simulation suggests that earlier initiation of combination antiretroviral therapy is often favored compared with current recommendations.
Conflict of interest statement
Figures

References
-
- Ho DD. Time to hit HIV, early and hard [Editorial] N Engl J Med. 1995;333:450–1. - PubMed
-
- Sulkowski MS, Thomas DL, Chaisson RE, Moore RD. Hepatotoxicity associated with antiretroviral therapy in adults infected with human immunodefi-ciency virus and the role of hepatitis C or B virus infection. JAMA. 2000;283:74–80. - PubMed
-
- Data Collection on Adverse Events of Anti-HIV Drugs (DAD) Study Group. Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med. 2003;349:1993–2003. - PubMed
-
- HIV Outpatient Study (HOPS) investigators. Protease inhibitors and cardiovascular outcomes in patients with HIV-1. Lancet. 2002;360:1747–8. - PubMed
-
- HIV-HCV Co-Infection Study Group. Severe hepatotoxicity during combination antiretroviral treatment: incidence, liver histology, and outcome. J Acquir Immune Defic Syndr. 2003;32:259–67. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
