Glycine transporter dimers: evidence for occurrence in the plasma membrane
- PMID: 18252709
- PMCID: PMC4503265
- DOI: 10.1074/jbc.M800622200
Glycine transporter dimers: evidence for occurrence in the plasma membrane
Abstract
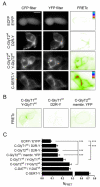
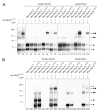
Different Na(+)/Cl(-)-dependent neurotransmitter transporters of the SLC6a family have been shown to form dimers or oligomers in both intracellular compartments and at the cell surface. In contrast, the glycine transporters (GlyTs) GlyT1 and -2 have been reported to exist as monomers in the plasma membrane based on hydrodynamic and native gel electrophoretic studies. Here, we used cysteine substitution and oxidative cross-linking to show that of GlyT1 and GlyT2 also form dimeric complexes within the plasma membrane. GlyT oligomerization at the cell surface was confirmed for both GlyT1 and GlyT2 by fluorescence resonance energy transfer microscopy. Endoglycosidase treatment and surface biotinylation further revealed that complex-glycosylated GlyTs form dimers located at the cell surface. Furthermore, substitution of tryptophan 469 of GlyT2 by an arginine generated a transporter deficient in dimerization that was retained intracellulary. Based on these results and GlyT structures modeled by using the crystal structure of the bacterial homolog LeuT(Aa), as a template, residues located within the extracellular loop 3 and at the beginning of transmembrane domain 6 are proposed to contribute to the dimerization interface of GlyTs.
Figures






References
-
- Torres GE, Gainetdinov RR, Caron MG. Nat. Rev. Neurosci. 2003;4:13–25. - PubMed
-
- Gether U, Andersen PH, Larsson OM, Schousboe A. Trends Pharmacol. Sci. 2006;27:375–383. - PubMed
-
- Eulenburg V, Armsen W, Betz H, Gomeza J. Trends Biochem. Sci. 2005;30:325–333. - PubMed
-
- Farhan H, Freissmuth M, Sitte HH. Handb. Exp. Pharmacol. 2006:233–249. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

