Enamel defects and ameloblast-specific expression in Enam knock-out/lacz knock-in mice
- PMID: 18252720
- PMCID: PMC2447669
- DOI: 10.1074/jbc.M710565200
Enamel defects and ameloblast-specific expression in Enam knock-out/lacz knock-in mice
Abstract
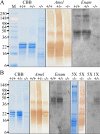
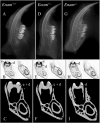
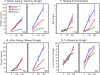
Enamelin is critical for proper dental enamel formation, and defects in the human enamelin gene cause autosomal dominant amelogenesis imperfecta. We used gene targeting to generate a knock-in mouse carrying a null allele of enamelin (Enam) that has a lacZ reporter gene replacing the Enam translation initiation site and gene sequences through exon 7. Correct targeting of the transgene was confirmed by Southern blotting and PCR analyses. No enamelin protein could be detected by Western blotting in the Enam-null mice. Histochemical 5-bromo-4-chloro-3-indolyl-beta-d-galactopyranoside (X-gal) staining demonstrated ameloblast-specific expression of enamelin. The enamel of the Enam(+/-) mice was nearly normal in the maxillary incisors, but the mandibular incisors were discolored and tended to wear rapidly where they contacted the maxillary incisors. The Enam(-/-) mice showed no true enamel. Radiography, microcomputed tomography, and light and scanning electron microscopy were used to document changes in the enamel of Enam(-/-) mice but did not discern any perturbations of bone, dentin, or any other tissue besides the enamel layer. Although a thick layer of enamel proteins covered normal-appearing dentin of unerupted teeth, von Kossa staining revealed almost a complete absence of mineral formation in this protein layer. However, a thin, highly irregular, mineralized crust covered the dentin on erupted teeth, apparently arising from the formation and fusion of small mineralization foci (calcospherites) in the deeper part of the accumulated enamel protein layer. These results demonstrate ameloblast-specific expression of enamelin and reveal that enamelin is essential for proper enamel matrix organization and mineralization.
Figures






References
-
- Daculsi, G., and Kerebel, B. (1978) J. Ultrastruct. Res. 65 163-172 - PubMed
-
- Nanci, A. (ed) (2003) in Ten Cate's Oral Histology Development, Structure, and Function, 6th Ed., pp. 218-224, Mosby, St. Louis, MO
-
- Fincham, A. G., Moradian-Oldak, J., and Simmer, J. P. (1999) J. Struct. Biol. 126 270-299 - PubMed
-
- Ronnholm, E. (1962) J. Ultrastruct. Res. 6 249-303 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

