Neurotensin in the ventral pallidum increases extracellular gamma-aminobutyric acid and differentially affects cue- and cocaine-primed reinstatement
- PMID: 18252810
- PMCID: PMC2672956
- DOI: 10.1124/jpet.107.130310
Neurotensin in the ventral pallidum increases extracellular gamma-aminobutyric acid and differentially affects cue- and cocaine-primed reinstatement
Abstract
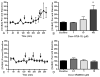
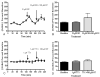
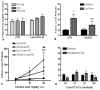
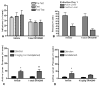
Cocaine-primed reinstatement is an animal model of drug relapse. The neurocircuitry underlying cocaine-primed reinstatement includes a decrease in GABA in the ventral pallidum (VP) that is inhibited by a mu opioid receptor antagonist, suggesting that opioid peptides colocalized with GABA in the projection from the nucleus accumbens to the VP may mediate this effect. Neurotensin is also colocalized with GABA and has been shown to increase GABA release in several brain regions. Therefore, the present study determined whether neurotensin increases GABA release in the VP, antagonizes cocaine-induced decreases in GABA, and prevents reinstatement of cocaine seeking. In vivo microdialysis revealed that the neurotensin agonist neurotensin peptide fragment 8-13 [NT(8-13)] increased GABA in the VP in a neurotensin receptor and tetrodotoxin-dependent manner and blocked the cocaine-induced decrease in GABA. NT(8-13) (3 nmol) microinjected into the VP prevented cue-induced reinstatement without affecting cocaine self-administration. In contrast, 3 nmol NT(8-13) potentiated cocaine-primed reinstatement. The neurotensin antagonist SR142948 (2-[[[5-(2,6-dimethoxyphenyl)-1-[4-[[[3-(dimethylamino)propyl]methylamino]carbonyl]-2-(1-methylethyl)phenyl]-1H -pyrazol-3-yl]carbonyl]amino]-tricyclo-[3.3.1.13,7]decane-2-carboxylic acid) had no effect on any behavioral measure when infused in the VP at the dose tested but attenuated cocaine-primed reinstatement when administered systemically. In contrast to reinstatement, NT(8-13) did not alter the motor response to acute cocaine or the development of motor sensitization by chronic cocaine. Three conclusions can be drawn from these data: 1) neurotensin promotes GABA release in the VP and correspondingly inhibits cue-induced reinstatement, 2) neurotensin and cocaine interact in a manner that countermands the neurotensin-induced increase in GABA and promotes reinstatement, and 3) endogenous release of neurotensin in the VP is not necessary for reinstatement.
Figures

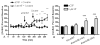
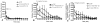

Similar articles
-
Cocaine-induced reinstatement requires endogenous stimulation of mu-opioid receptors in the ventral pallidum.J Neurosci. 2005 May 4;25(18):4512-20. doi: 10.1523/JNEUROSCI.0685-05.2005. J Neurosci. 2005. PMID: 15872098 Free PMC article.
-
The glutamatergic projection from the prefrontal cortex to the nucleus accumbens core is required for cocaine-induced decreases in ventral pallidal GABA.Neurosci Lett. 2008 Jun 20;438(2):142-5. doi: 10.1016/j.neulet.2008.04.016. Epub 2008 Apr 10. Neurosci Lett. 2008. PMID: 18455875 Free PMC article.
-
Cannabinoid CB1 receptor antagonist AM251 inhibits cocaine-primed relapse in rats: role of glutamate in the nucleus accumbens.J Neurosci. 2006 Aug 16;26(33):8531-6. doi: 10.1523/JNEUROSCI.0726-06.2006. J Neurosci. 2006. PMID: 16914679 Free PMC article.
-
Differential effects of intrastriatal neurotensin(1-13) and neurotensin(8-13) on striatal dopamine and pallidal GABA release. A dual-probe microdialysis study in the awake rat.Eur J Neurosci. 1997 Sep;9(9):1838-46. doi: 10.1111/j.1460-9568.1997.tb00750.x. Eur J Neurosci. 1997. PMID: 9383206
-
Metabotropic glutamate 7 (mGlu7) receptor: a target for medication development for the treatment of cocaine dependence.Neuropharmacology. 2013 Mar;66:12-23. doi: 10.1016/j.neuropharm.2012.04.010. Epub 2012 Apr 21. Neuropharmacology. 2013. PMID: 22546614 Free PMC article. Review.
Cited by
-
Response of neurotensin basal ganglia systems during extinction of methamphetamine self-administration in rat.J Pharmacol Exp Ther. 2013 Aug;346(2):173-81. doi: 10.1124/jpet.113.205310. Epub 2013 May 17. J Pharmacol Exp Ther. 2013. PMID: 23685547 Free PMC article.
-
Ventral pallidal regulation of motivated behaviors and reinforcement.Front Neural Circuits. 2023 Feb 2;17:1086053. doi: 10.3389/fncir.2023.1086053. eCollection 2023. Front Neural Circuits. 2023. PMID: 36817646 Free PMC article. Review.
-
Metabotropic glutamate receptor 7 modulates the rewarding effects of cocaine in rats: involvement of a ventral pallidal GABAergic mechanism.Neuropsychopharmacology. 2009 Jun;34(7):1783-96. doi: 10.1038/npp.2008.236. Epub 2009 Jan 21. Neuropsychopharmacology. 2009. PMID: 19158667 Free PMC article.
-
Double dissociation between the anterior cingulate cortex and nucleus accumbens core in encoding the context versus the content of pavlovian cocaine cue extinction.J Neurosci. 2013 May 8;33(19):8370-7. doi: 10.1523/JNEUROSCI.0489-13.2013. J Neurosci. 2013. PMID: 23658176 Free PMC article.
-
Changes in dorsomedial striatum activity during expression of goal-directed vs. habit-like cue-induced cocaine seeking.Addict Neurosci. 2024 Jun;11:100149. doi: 10.1016/j.addicn.2024.100149. Epub 2024 Jan 28. Addict Neurosci. 2024. PMID: 38957402 Free PMC article.
References
-
- Bayer VE, Towle AC, Pickel VM. Ultrastructural localization of neurotensin-like immunoreactivity within dense core vesicles in perikarya, but not terminals, colocalizing tyrosine hydroxylase in the rat ventral tegmental area. J Comp Neurol. 1991;311:179–196. - PubMed
-
- Binder EB, Gross RE, Nemeroff CB, Kilts CD. Effects of neurotensin receptor antagonism on latent inhibition in Sprague-Dawley rats. Psychopharmacology. 2002;161:288–295. - PubMed
-
- Boules M, Warrington L, Fauq A, McCormick D, Richelson E. A novel neurotensin analog blocks cocaine- and D-amphetamine-induced hyperactivity. Eur J Pharmacol. 2001;426:73–76. - PubMed
-
- Bourdelais A, Kalivas PW. Amphetamine lowers extracellular GABA concentration in the ventral pallidum. Brain Res. 1990;516:132–136. - PubMed
-
- Cáceda R, Kinkead B, Nemeroff CB. Neurotensin: role in psychiatric and neurological diseases. Peptides. 2006;27:2385–2404. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

