20(S)-25-methoxyl-dammarane-3beta, 12beta, 20-triol, a novel natural product for prostate cancer therapy: activity in vitro and in vivo and mechanisms of action
- PMID: 18253123
- PMCID: PMC2259179
- DOI: 10.1038/sj.bjc.6604227
20(S)-25-methoxyl-dammarane-3beta, 12beta, 20-triol, a novel natural product for prostate cancer therapy: activity in vitro and in vivo and mechanisms of action
Abstract
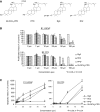
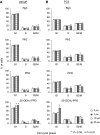
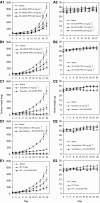
We recently isolated 20(S)-25-methoxyl-dammarane-3beta, 12beta, 20-triol (25-OCH3-PPD), a natural product from Panax notoginseng, and demonstrated its cytotoxicity against a variety of cancer cells. Here we report the effects of this compound in vitro and in vivo on human prostate cancer cells, LNCaP (androgen-dependent) and PC3 (androgen-independent), in comparison with three structurally related ginsenosides, ginsenoside Rh2, ginsenoside Rg3, and 20(S)-protopanaxadiol. Of the four test compounds, 25-OCH3-PPD was most potent. It decreased survival, inhibited proliferation, induced apoptosis, and led to G1 cell cycle arrest in both cell lines. It also decreased the levels of proteins associated with cell proliferation (MDM2, E2F1, cyclin D1, and cdks 2 and 4) and increased or activated pro-apoptotic proteins (cleaved PARP, cleaved caspase-3, -8, and -9). In LNCaP cells, 25-OCH3-PPD inhibited the expression of the androgen receptor and prostate-specific antigen. Moreover, 25-OCH3-PPD inhibited the growth of prostate cancer xenograft tumours. Combining 25-OCH3-PPD with conventional chemotherapeutic agents or with radiation led to potent antitumour effects; tumour regression was almost complete following administration of 25-OCH3-PPD and either taxotere or gemcitabine. 25-OCH3-PPD also demonstrated low toxicity to noncancer cells and no observable toxicity in animals. In conclusion, our preclinical data indicate that 25-OCH3-PPD is a potential therapeutic agent against both androgen-dependent and androgen-independent prostate cancer.
Figures



Similar articles
-
Natural product ginsenoside 25-OCH3-PPD inhibits breast cancer growth and metastasis through down-regulating MDM2.PLoS One. 2012;7(7):e41586. doi: 10.1371/journal.pone.0041586. Epub 2012 Jul 23. PLoS One. 2012. PMID: 22911819 Free PMC article.
-
Isolation, structural determination, and evaluation of the biological activity of 20(S)-25-methoxyl-dammarane-3beta, 12beta, 20-triol [20(S)-25-OCH3-PPD], a novel natural product from Panax notoginseng.Med Chem. 2007 Jan;3(1):51-60. doi: 10.2174/157340607779317508. Med Chem. 2007. PMID: 17266624
-
Experimental therapy of prostate cancer with novel natural product anti-cancer ginsenosides.Prostate. 2008 Jun 1;68(8):809-19. doi: 10.1002/pros.20742. Prostate. 2008. PMID: 18324646
-
Natural Product Ginsenoside 20(S)-25-Methoxyl-Dammarane-3β, 12β, 20-Triol in Cancer Treatment: A Review of the Pharmacological Mechanisms and Pharmacokinetics.Front Pharmacol. 2020 Apr 22;11:521. doi: 10.3389/fphar.2020.00521. eCollection 2020. Front Pharmacol. 2020. PMID: 32425780 Free PMC article. Review.
-
Pharmacokinetics and pharmacodynamics of Rh2 and aPPD ginsenosides in prostate cancer: a drug interaction perspective.Cancer Chemother Pharmacol. 2023 Dec;92(6):419-437. doi: 10.1007/s00280-023-04583-y. Epub 2023 Sep 15. Cancer Chemother Pharmacol. 2023. PMID: 37709921 Review.
Cited by
-
Saponins from Chinese Medicines as Anticancer Agents.Molecules. 2016 Oct 5;21(10):1326. doi: 10.3390/molecules21101326. Molecules. 2016. PMID: 27782048 Free PMC article. Review.
-
20(S)-protopanaxadiol regio-selectively targets androgen receptor: anticancer effects in castration-resistant prostate tumors.Oncotarget. 2018 Apr 20;9(30):20965-20978. doi: 10.18632/oncotarget.24695. eCollection 2018 Apr 20. Oncotarget. 2018. PMID: 29765513 Free PMC article.
-
Natural product ginsenoside 25-OCH3-PPD inhibits breast cancer growth and metastasis through down-regulating MDM2.PLoS One. 2012;7(7):e41586. doi: 10.1371/journal.pone.0041586. Epub 2012 Jul 23. PLoS One. 2012. PMID: 22911819 Free PMC article.
-
JKA97, a novel benzylidene analog of harmine, exerts anti-cancer effects by inducing G1 arrest, apoptosis, and p53-independent up-regulation of p21.PLoS One. 2012;7(4):e34303. doi: 10.1371/journal.pone.0034303. Epub 2012 Apr 27. PLoS One. 2012. PMID: 22558087 Free PMC article.
-
Development and validation of a rapid HPLC method for quantitation of SP-141, a novel pyrido[b]indole anticancer agent, and an initial pharmacokinetic study in mice.Biomed Chromatogr. 2015 May;29(5):654-63. doi: 10.1002/bmc.3327. Epub 2014 Oct 8. Biomed Chromatogr. 2015. PMID: 25294254 Free PMC article.
References
-
- American Cancer Society, Inc. (2007) Leading sites of new cancer cases and deaths-2007 estimates. surveillance research 2007. Available online: http://www.cancer.org/downloads/STT/CAFF2007PWSecured.pdf Site accessed November 11, 2007
-
- Attele AS, Wu JA, Yuan CS (1999) Ginseng pharmacology: multiple constituents and multiple actions. Biochem Pharmacol 58: 1685–1693. DOI: 10.1016/S0006-2952(99)00212-9 - PubMed
-
- Bosland MC, McCormick DL, Melamed J, Walden PD, Zeleniuch-Jacquotte A, Lumey LH (2002) Chemoprevention strategies for prostate cancer. Eur J Cancer Prev 11(Suppl 2): S18–S27 - PubMed
-
- Cheng CC, Yang SM, Huang CY, Chen JC, Chang WM, Hsu SL (2005) Molecular mechanisms of ginsenoside Rh2-mediated G1 growth arrest and apoptosis in human lung adenocarcinoma A549 cells. Cancer Chemother Pharmacol 55: 531–540. DOI: 10.1007/s00280-004-0919-6 - PubMed
-
- Cho SD, Jiang C, Malewicz B, Dong Y, Young CY, Kang KS, Lee YS, Ip C, Lu J (2004) Methyl selenium metabolites decrease prostate-specific antigen expression by inducing protein degradation and suppressing androgen-stimulated transcription. Mol Cancer Ther 3: 605–611 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

