Commercial nucleic-acid amplification tests for diagnosis of pulmonary tuberculosis in respiratory specimens: meta-analysis and meta-regression
- PMID: 18253484
- PMCID: PMC2212137
- DOI: 10.1371/journal.pone.0001536
Commercial nucleic-acid amplification tests for diagnosis of pulmonary tuberculosis in respiratory specimens: meta-analysis and meta-regression
Abstract
Background: Hundreds of studies have evaluated the diagnostic accuracy of nucleic-acid amplification tests (NAATs) for tuberculosis (TB). Commercial tests have been shown to give more consistent results than in-house assays. Previous meta-analyses have found high specificity but low and highly variable estimates of sensitivity. However, reasons for variability in study results have not been adequately explored. We performed a meta-analysis on the accuracy of commercial NAATs to diagnose pulmonary TB and meta-regression to identify factors that are associated with higher accuracy.
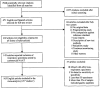
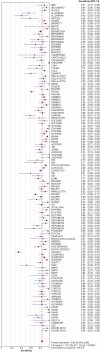
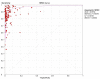
Methodology/principal findings: We identified 2948 citations from searching the literature. We found 402 articles that met our eligibility criteria. In the final analysis, 125 separate studies from 105 articles that reported NAAT results from respiratory specimens were included. The pooled sensitivity was 0.85 (range 0.36-1.00) and the pooled specificity was 0.97 (range 0.54-1.00). However, both measures were significantly heterogeneous (p<.001). We performed subgroup and meta-regression analyses to identify sources of heterogeneity. Even after stratifying by type of commercial test, we could not account for the variability. In the meta-regression, the threshold effect was significant (p = .01) and the use of other respiratory specimens besides sputum was associated with higher accuracy.
Conclusions/significance: The sensitivity and specificity estimates for commercial NAATs in respiratory specimens were highly variable, with sensitivity lower and more inconsistent than specificity. Thus, summary measures of diagnostic accuracy are not clinically meaningful. The use of different cut-off values and the use of specimens other than sputum could explain some of the observed heterogeneity. Based on these observations, commercial NAATs alone cannot be recommended to replace conventional tests for diagnosing pulmonary TB. Improvements in diagnostic accuracy, particularly sensitivity, need to be made in order for this expensive technology to be worthwhile and beneficial in low-resource countries.
Conflict of interest statement
Figures

References
-
- WHO. Geneva, Switzerland: WHO; 2006. Global Tuberculosis Control: Surveillance, Planning, Financing. pp. 1–242. WHO/HTM/TB/2006.362 WHO/HTM/TB/2006.362.
-
- Dye C, Scheele S, Dolin P, Pathania V, Raviglione MC. Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. WHO Global Surveillance and Monitoring Project. Jama. 1999;282:677–686. - PubMed
-
- Steingart KR, Henry M, Ng V, Hopewell PC, Ramsay A, et al. Fluorescence versus conventional sputum smear microscopy for tuberculosis: a systematic review. Lancet Infect Dis. 2006;6:570–581. - PubMed
-
- Steingart KR, Ng V, Henry M, Hopewell PC, Ramsay A, et al. Sputum processing methods to improve the sensitivity of smear microscopy for tuberculosis: a systematic review. Lancet Infect Dis. 2006;6:664–674. - PubMed
-
- Pai M. The accuracy and reliability of nucleic acid amplification tests in the diagnosis of tuberculosis. Natl Med J India. 2004;17:233–236. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

