Establishment of mouse embryonic stem cell-derived erythroid progenitor cell lines able to produce functional red blood cells
- PMID: 18253492
- PMCID: PMC2212133
- DOI: 10.1371/journal.pone.0001544
Establishment of mouse embryonic stem cell-derived erythroid progenitor cell lines able to produce functional red blood cells
Abstract
Background: The supply of transfusable red blood cells (RBCs) is not sufficient in many countries. If erythroid cell lines able to produce transfusable RBCs in vitro were established, they would be valuable resources. However, such cell lines have not been established. To evaluate the feasibility of establishing useful erythroid cell lines, we attempted to establish such cell lines from mouse embryonic stem (ES) cells.
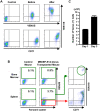
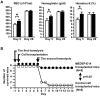
Methodology/principal findings: We developed a robust method to obtain differentiated cell lines following the induction of hematopoietic differentiation of mouse ES cells and established five independent hematopoietic cell lines using the method. Three of these lines exhibited characteristics of erythroid cells. Although their precise characteristics varied, each of these lines could differentiate in vitro into more mature erythroid cells, including enucleated RBCs. Following transplantation of these erythroid cells into mice suffering from acute anemia, the cells proliferated transiently, subsequently differentiated into functional RBCs, and significantly ameliorated the acute anemia. In addition, we did not observe formation of any tumors following transplantation of these cells.
Conclusion/significance: To the best of our knowledge, this is the first report to show the feasibility of establishing erythroid cell lines able to produce mature RBCs. Considering the number of human ES cell lines that have been established so far, the intensive testing of a number of these lines for erythroid potential may allow the establishment of human erythroid cell lines similar to the mouse erythroid cell lines described here. In addition, our results strongly suggest the possibility of establishing useful cell lines committed to specific lineages other than hematopoietic progenitors from human ES cells.
Conflict of interest statement
Figures


Similar articles
-
Red blood cell production from immortalized progenitor cell line.Int J Hematol. 2011 Jan;93(1):5-9. doi: 10.1007/s12185-010-0742-2. Epub 2010 Dec 25. Int J Hematol. 2011. PMID: 21184289 Review.
-
Establishment of immortalized human erythroid progenitor cell lines able to produce enucleated red blood cells.PLoS One. 2013;8(3):e59890. doi: 10.1371/journal.pone.0059890. Epub 2013 Mar 22. PLoS One. 2013. PMID: 23533656 Free PMC article.
-
Plasticity of cells and ex vivo production of red blood cells.Stem Cells Int. 2011;2011:195780. doi: 10.4061/2011/195780. Epub 2011 Jul 7. Stem Cells Int. 2011. PMID: 21785608 Free PMC article.
-
Establishment of an erythroid progenitor cell line capable of enucleation achieved with an inducible c-Myc vector.BMC Biotechnol. 2019 Apr 15;19(1):21. doi: 10.1186/s12896-019-0515-9. BMC Biotechnol. 2019. PMID: 30987611 Free PMC article.
-
From stem cells to red blood cells: how far away from the clinical application?Sci China Life Sci. 2014 Jun;57(6):581-5. doi: 10.1007/s11427-014-4667-5. Epub 2014 May 15. Sci China Life Sci. 2014. PMID: 24829108 Review.
Cited by
-
Stem cell-derived erythrocytes as upcoming players in blood transfusion.ISBT Sci Ser. 2013 Jun;8(1):165-171. doi: 10.1111/voxs.12048. ISBT Sci Ser. 2013. PMID: 26229549 Free PMC article.
-
APOA-1 is a novel marker of erythroid cell maturation from hematopoietic stem cells in mice and humans.Stem Cell Rev Rep. 2011 Mar;7(1):43-52. doi: 10.1007/s12015-010-9140-7. Stem Cell Rev Rep. 2011. PMID: 20376577
-
Manufacturing blood ex vivo: a futuristic approach to deal with the supply and safety concerns.Front Cell Dev Biol. 2014 Jun 11;2:26. doi: 10.3389/fcell.2014.00026. eCollection 2014. Front Cell Dev Biol. 2014. PMID: 25364733 Free PMC article. Review.
-
GATA2 and PU.1 Collaborate To Activate the Expression of the Mouse Ms4a2 Gene, Encoding FcεRIβ, through Distinct Mechanisms.Mol Cell Biol. 2019 Oct 28;39(22):e00314-19. doi: 10.1128/MCB.00314-19. Print 2019 Nov 15. Mol Cell Biol. 2019. PMID: 31501274 Free PMC article.
-
Induced pluripotent stem (iPS) cells offer a powerful new tool for the life sciences.J Stem Cells Regen Med. 2010 Apr 5;6(1):2-9. doi: 10.46582/jsrm.0601002. eCollection 2010. J Stem Cells Regen Med. 2010. PMID: 24693054 Free PMC article.
References
-
- Neildez-Nguyen TM, Wajcman H, Marden MC, Bensidhoum M, Moncollin V, et al. Human erythroid cells produced ex vivo at large scale differentiate into red blood cells in vivo. Nat Biotechnol. 2002;20:467–472. - PubMed
-
- Giarratana MC, Kobari L, Lapillonne H, Chalmers D, Kiger L, et al. Ex vivo generation of fully mature human red blood cells from hematopoietic stem cells. Nat Biotechnol. 2005;23:69–74. - PubMed
-
- Miharada K, Hiroyama T, Sudo K, Nagasawa T, Nakamura Y. Efficient enucleation of erythroblasts differentiated in vitro from hematopoietic stem and progenitor cells. Nat Biotechnol. 2006;24:1255–1256. - PubMed
-
- Nakano T, Kodama H, Honjo T. Generation of lymphohematopoietic cells from embryonic stem cells in culture. Science. 1994;265:1098–1101. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

