The Plasmodium export element revisited
- PMID: 18253504
- PMCID: PMC2216060
- DOI: 10.1371/journal.pone.0001560
The Plasmodium export element revisited
Abstract
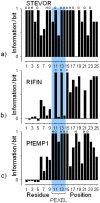
We performed a bioinformatical analysis of protein export elements (PEXEL) in the putative proteome of the malaria parasite Plasmodium falciparum. A protein family-specific conservation of physicochemical residue profiles was found for PEXEL-flanking sequence regions. We demonstrate that the family members can be clustered based on the flanking regions only and display characteristic hydrophobicity patterns. This raises the possibility that the flanking regions may contain additional information for a family-specific role of PEXEL. We further show that signal peptide cleavage results in a positional alignment of PEXEL from both proteins with, and without, a signal peptide.
Conflict of interest statement
Figures



References
-
- Cooke BM, Lingelbach K, Bannister LH, Tilley L. Protein trafficking in Plasmodium falciparum-infected red blood cells. Trends Parasitol. 2004;20:581–589. - PubMed
-
- Hiller NL, Bhattacharjee S, van Ooij C, Liolios K, Harrison T, et al. A host-targeting signal in virulence proteins reveals a secretome in malarial infection. Science. 2004;306:1934–1937. - PubMed
-
- Marti M, Good RT, Rug M, Knuepfer E, Cowman AF. Targeting malaria virulence and remodeling proteins to the host erythrocyte. Science. 2004;306:1930–1933. - PubMed
-
- Baruch DI, Pasloske BL, Singh HB, Bi X, Ma XC, et al. Cloning the P. falciparum gene encoding PfEMP1, a malarial variant antigen and adherence receptor on the surface of parasitized human erythrocytes. Cell. 1995;82:77–87. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

