Group III mGluR regulation of synaptic transmission at the SC-CA1 synapse is developmentally regulated
- PMID: 18255102
- PMCID: PMC2431171
- DOI: 10.1016/j.neuropharm.2007.12.009
Group III mGluR regulation of synaptic transmission at the SC-CA1 synapse is developmentally regulated
Abstract
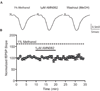
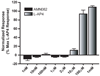
Group III metabotropic glutamate receptors (mGluRs) reduce synaptic transmission at the Schaffer collateral-CA1 (SC-CA1) synapse in rats by a presynaptic mechanism. Previous studies show that low concentrations of the group III-selective agonist, L-AP4, reduce synaptic transmission in slices from neonatal but not adult rats, whereas high micromolar concentrations reduce transmission in both age groups. L-AP4 activates mGluRs 4 and 8 at much lower concentrations than those required to activate mGluR7, suggesting that the group III mGluR subtype modulating transmission is a high affinity receptor in neonates and a low affinity receptor in adults. The previous lack of subtype selective ligands has made it difficult to test this hypothesis. We have measured fEPSPs in the presence of novel subtype selective agents to address this question. We show that the effects of L-AP4 can be blocked by LY341495 in both neonates and adults, verifying that these effects are mediated by mGluRs. In addition, the selective mGluR8 agonist, DCPG, has a significant effect in slices from neonatal rats but does not reduce synaptic transmission in adult slices. The mGluR4 selective allosteric potentiator, PHCCC, is unable to potentiate the L-AP4-induced effects at either age. Taken together, our data suggest that group III mGluRs regulate transmission at the SC-CA1 synapse throughout development but there is a developmental regulation of the subtypes involved so that both mGluR7 and mGluR8 serve this role in neonates whereas mGluR7 is involved in regulating transmission at this synapse throughout postnatal development.
Figures
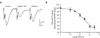
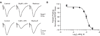





Similar articles
-
Metabotropic glutamate receptors mGluR4 and mGluR8 regulate transmission in the lateral olfactory tract-piriform cortex synapse.Neuropharmacology. 2008 Sep;55(4):440-6. doi: 10.1016/j.neuropharm.2008.06.043. Epub 2008 Jun 29. Neuropharmacology. 2008. PMID: 18625254 Free PMC article.
-
Metabotropic glutamate receptor 2 modulates excitatory synaptic transmission in the rat globus pallidus.Neuropharmacology. 2005;49 Suppl 1:57-69. doi: 10.1016/j.neuropharm.2005.03.006. Neuropharmacology. 2005. PMID: 15993439
-
Differential involvement of group II and group III mGluRs as autoreceptors at lateral and medial perforant path synapses.J Neurophysiol. 1996 Dec;76(6):3798-806. doi: 10.1152/jn.1996.76.6.3798. J Neurophysiol. 1996. PMID: 8985877
-
Multiple presynaptic metabotropic glutamate receptors modulate excitatory and inhibitory synaptic transmission in hippocampal area CA1.J Neurosci. 1995 Oct;15(10):6879-89. doi: 10.1523/JNEUROSCI.15-10-06879.1995. J Neurosci. 1995. PMID: 7472445 Free PMC article.
-
Modulation of excitatory synaptic transmission by endogenous glutamate acting on presynaptic group II mGluRs in rat substantia nigra compacta.J Neurosci Res. 2005 Dec 15;82(6):778-87. doi: 10.1002/jnr.20694. J Neurosci Res. 2005. PMID: 16273546
Cited by
-
Activation of Metabotropic Glutamate Receptor 7 Is Required for Induction of Long-Term Potentiation at SC-CA1 Synapses in the Hippocampus.J Neurosci. 2015 May 13;35(19):7600-15. doi: 10.1523/JNEUROSCI.4543-14.2015. J Neurosci. 2015. PMID: 25972184 Free PMC article.
-
Aβ selectively impairs mGluR7 modulation of NMDA signaling in basal forebrain cholinergic neurons: implication in Alzheimer's disease.J Neurosci. 2014 Oct 8;34(41):13614-28. doi: 10.1523/JNEUROSCI.1204-14.2014. J Neurosci. 2014. PMID: 25297090 Free PMC article.
-
Detailed In Vitro Pharmacological Characterization of Clinically Tested Negative Allosteric Modulators of the Metabotropic Glutamate Receptor 5.Mol Pharmacol. 2020 Jul;98(1):49-60. doi: 10.1124/mol.119.119032. Epub 2020 May 1. Mol Pharmacol. 2020. PMID: 32358164 Free PMC article.
-
Molecular Insights into Metabotropic Glutamate Receptor Allosteric Modulation.Mol Pharmacol. 2015 Jul;88(1):188-202. doi: 10.1124/mol.114.097220. Epub 2015 Mar 25. Mol Pharmacol. 2015. PMID: 25808929 Free PMC article.
-
Metabotropic Glutamate Receptor 7: A New Therapeutic Target in Neurodevelopmental Disorders.Front Mol Neurosci. 2018 Oct 23;11:387. doi: 10.3389/fnmol.2018.00387. eCollection 2018. Front Mol Neurosci. 2018. PMID: 30405350 Free PMC article. Review.
References
-
- Bradley SR, Rees HD, Yi H, Levey AI, Conn PJ. Distribution and developmental regulation of metabotropic glutamate receptor 7a in rat brain. J Neurochem. 1998;71:636–645. - PubMed
-
- Canudas AM, Di Giorgi-Gerevini V, Iacovelli L, Nano G, D'Onofrio M, Arcella A, Giangaspero F, Busceti C, Ricci-Vitiani L, Battaglia G, Nicoletti F, Melchiorri D. PHCCC, a specific enhancer of type 4 metabotropic glutamate receptors, reduces proliferation and promotes differentiation of cerebellar granule cell neuroprecursors. J Neurosci. 2004;24:10343–10352. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

