A trap for in situ cultivation of filamentous actinobacteria
- PMID: 18255181
- PMCID: PMC2293972
- DOI: 10.1016/j.mimet.2007.12.009
A trap for in situ cultivation of filamentous actinobacteria
Abstract
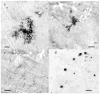
The approach of growing microorganisms in situ, or in a simulated natural environment is appealing, and different versions of it have been described by several groups. The major difficulties with these approaches are that they are not selective for actinomycetes - a group of gram-positive bacteria well known as a rich source of antibiotics. In order to efficiently access actinomycetes, a trap for specifically capturing and cultivating these microorganisms in situ has been developed, based on the ability of these bacteria to form hyphae and penetrate solid environments. The trap is formed by two semi-permeable membranes (0.2-0.6 microm pore-size bottom membrane and 0.03 microm pore-size top membrane) glued to a plastic washer with sterile agar or gellan gum inside. The trap is placed on top of soil, and filamentous microorganisms selectively penetrate into the device and form colonies. Decreasing the size of the pores of the lower membrane to 0.2 microm restricted penetration of fungi. The trap produced more filamentous actinobacteria, and a higher variety of them, as compared to a conventional Petri dish cultivation from the same soil sample. Importantly, the trap cultivation resulted in the isolation of unusual and rare actinomycetes.
Figures


References
-
- Baltz RH. Marcel Faber Roundtable: is our antibiotic pipeline unproductive because of starvation, constipation or lack of inspiration? J Ind Microbiol Biotechnol. 2006;33:507–513. - PubMed
-
- Busti EL, Cavaletti P, Monciardini P, Schumann M, Rohde M, Sosio S, Donadio Catenulispora acidiphila gen. nov., sp. nov., a novel mycelium-forming actinomycete and proposal of Catenulisporaceae fam. nov. Int J Syst Evol Microbiol. 2006;56:1741–1746. - PubMed
-
- Demain AL, Fang A. The natural functions of secondary metabolites. In: Fiechter IA, editor. History of Modern Biotechnology. Springer; Berlin: 2000. pp. 1–39. - PubMed
-
- Hopwood DA. Soil to genomics: the Streptomyces chromosome. Annu Rev Genet. 2006;40:1–23. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

