Increased sulfate uptake by E. coli overexpressing the SLC26-related SulP protein Rv1739c from Mycobacterium tuberculosis
- PMID: 18255326
- PMCID: PMC2712756
- DOI: 10.1016/j.cbpa.2007.12.005
Increased sulfate uptake by E. coli overexpressing the SLC26-related SulP protein Rv1739c from Mycobacterium tuberculosis
Abstract
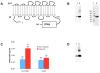
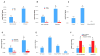
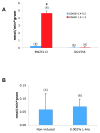
Growth and virulence of mycobacteria requires sulfur uptake. The Mycobacterium tuberculosis genome contains, in addition to the ABC sulfate permease cysTWA, three SLC26-related SulP genes of unknown function. We report that induction of Rv1739c expression in E. coli increased bacterial uptake of sulfate, but not Cl(-), formate, or oxalate. Uptake was time-dependent, maximal at pH 6.0, and exhibited a K(1/2) for sulfate of 4.0 muM. Na(+)-independent sulfate uptake was not reduced by bicarbonate, nitrate, or phosphate, but was inhibited by sulfite, selenate, thiosulfate, N-ethylmaleimide and carbonyl cyanide 3-chloro-phenylhydrazone. Sulfate uptake was also increased by overexpression of the Rv1739c transmembrane domain, but not of the cytoplasmic C-terminal STAS domain. Mutation to serine of the three cysteine residues of Rv1739c did not affect magnitude, pH-dependence, or pharmacology of sulfate uptake. Expression of Rv1739c in a M. bovis BCG strain lacking the ABC sulfate permease subunit CysA could not complement sulfate auxotrophy. Moreover, inducible expression of Rv1739c in an E. coli strain lacking CysA did not increase sulfate uptake by intact cells. Our data show that facilitation of bacterial sulfate uptake by Rv1739c requires CysA and its associated sulfate permease activity, and suggest that Rv1739c may be a CysTWA-dependent sulfate transporter.
Figures



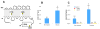

References
-
- Alper SL. Molecular physiology of SLC4 anion exchangers. Exp Physiol. 2006;91:153–161. - PubMed
-
- Arifuzzaman M, Maeda M, Itoh A, Nishikata K, Takita C, Saito R, Ara T, Nakahigashi K, Huang HC, Hirai A, Tsuzuki K, Nakamura S, Altaf-Ul-Amin M, Oshima T, Baba T, Yamamoto N, Kawamura T, Ioka-Nakamichi T, Kitagawa M, Tomita M, Kanaya S, Wada C, Mori H. Large-scale identification of protein-protein interaction of Escherichia coli K-12. Genome Res. 2006;16:686–691. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

